Abstract
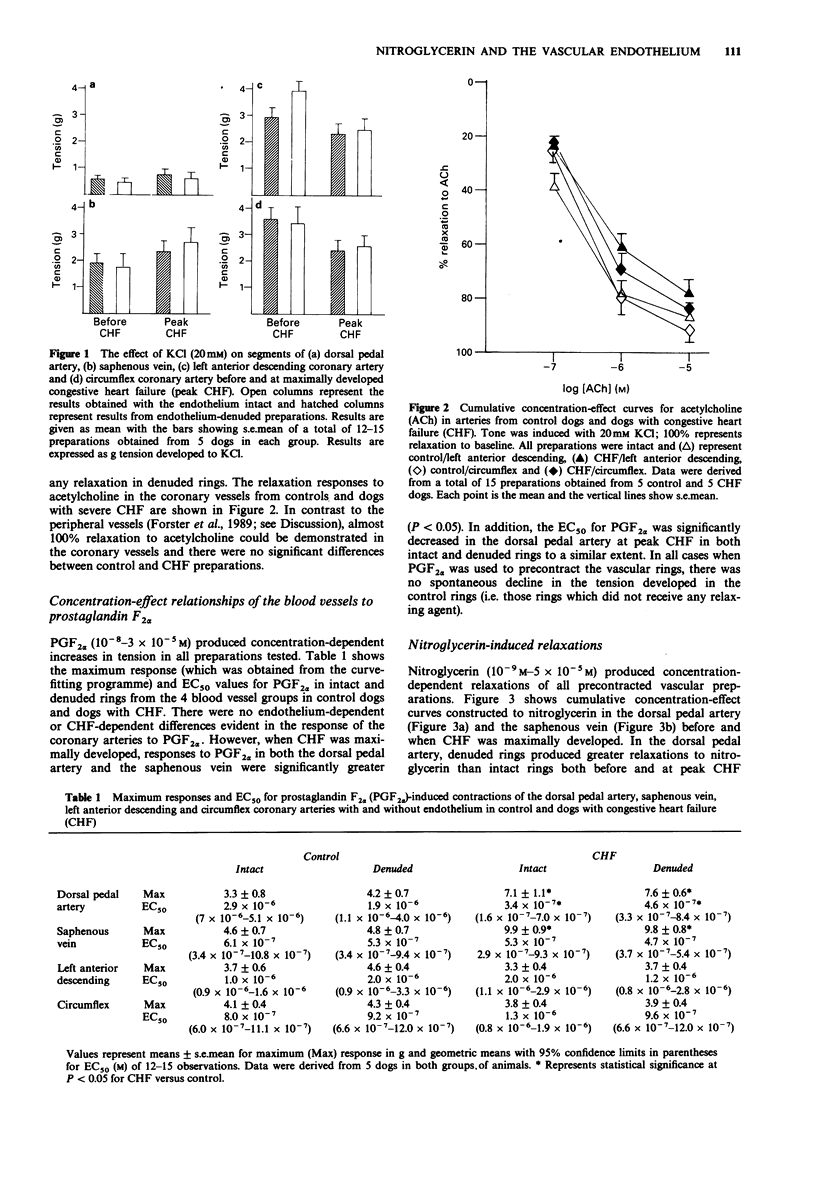
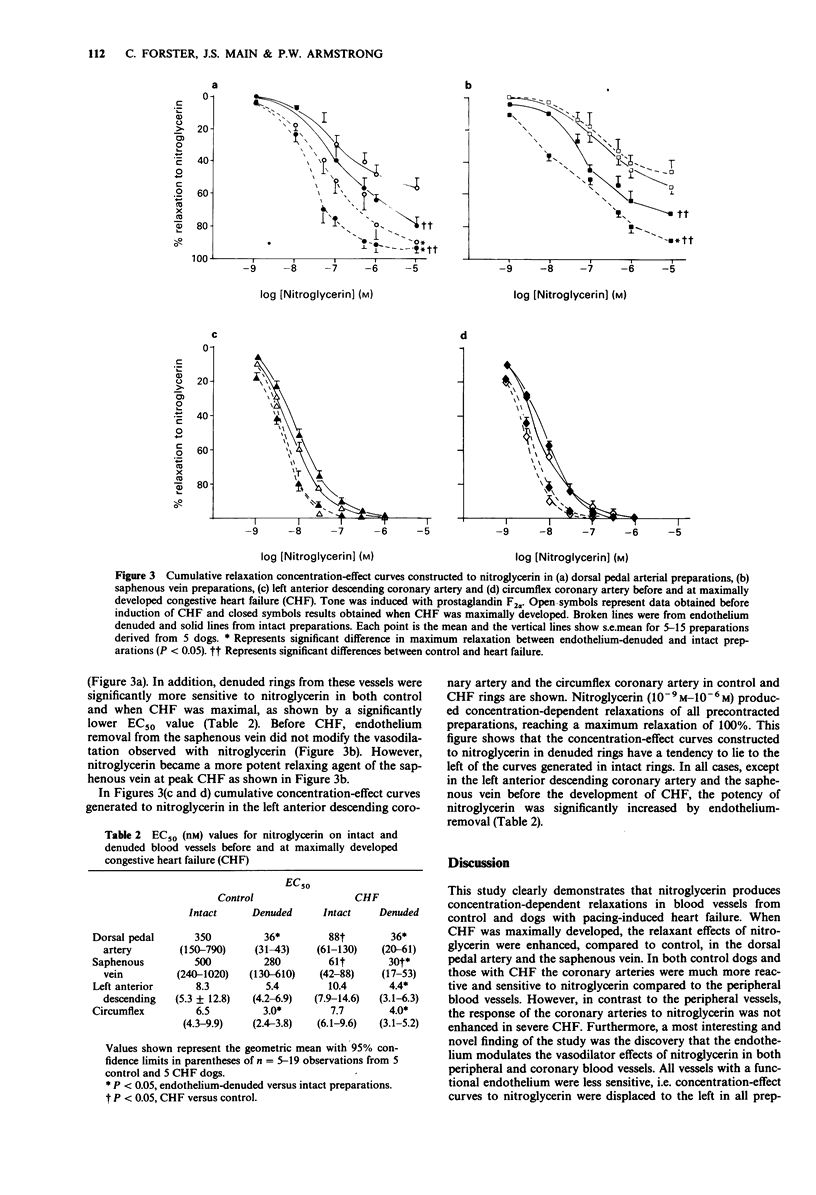
1. The relaxant actions of nitroglycerin (previously considered to be an endothelium-independent relaxing agent) and acetylcholine (an endothelium-dependent relaxing agent) were compared on 4 vascular preparations (dorsal pedal artery, saphenous vein, left anterior descending coronary artery and circumflex coronary artery) from dogs with and without pacing-induced congestive heart failure (CHF). 2. Responses of the coronary arteries to acetylcholine were unaltered in endothelium-intact rings from dogs with and without heart failure. Similarly no such changes were observed in the peripheral vessels. The maximum relaxation produced by acetylcholine was always greater in the coronary vessels compared to the peripheral vessels. 3. Before heart failure, the coronary vessels were more sensitive and reactive to nitroglycerin compared to the peripheral vessels. 4. Removal of the endothelium in both the control (dogs without CHF) and experimental (dogs with CHF) rings enhanced the relaxant effects of nitroglycerin, such that the EC50 for nitroglycerin became significantly lower in all denuded rings, with the exception of the saphenous vein and the left anterior descending coronary artery, before the development of CHF. 5. When CHF was maximally developed, vascular sensitivity to nitroglycerin was increased in peripheral vessels with an intact endothelium, but not in the coronary vessels. 6. These findings indicate that relaxation produced by nitroglycerin cannot be considered as entirely endothelium-independent but should be considered endothelium-modulated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdollah A., Moffat J. A., Armstrong P. W. N-acetylcysteine does not modify nitroglycerin-induced tolerance in canine vascular rings. J Cardiovasc Pharmacol. 1987 Apr;9(4):445–450. doi: 10.1097/00005344-198704000-00009. [DOI] [PubMed] [Google Scholar]
- Armstrong P. W., Stopps T. P., Ford S. E., de Bold A. J. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation. 1986 Nov;74(5):1075–1084. doi: 10.1161/01.cir.74.5.1075. [DOI] [PubMed] [Google Scholar]
- Armstrong P. W., Walker D. C., Burton J. R., Parker J. O. Vasodilator therapy in acute myocardial infarction. A comparison of sodium nitroprusside and nitroglycerin. Circulation. 1975 Dec;52(6):1118–1122. doi: 10.1161/01.cir.52.6.1118. [DOI] [PubMed] [Google Scholar]
- Aub D. L., Putney J. W., Jr Properties of receptor-controlled inositol trisphosphate formation in parotid acinar cells. Biochem J. 1985 Jan 1;225(1):263–266. doi: 10.1042/bj2250263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson K. L., Andersson R. G. Tolerance towards nitroglycerin, induced in vivo, is correlated to a reduced cGMP response and an alteration in cGMP turnover. Eur J Pharmacol. 1983 Mar 18;88(1):71–79. doi: 10.1016/0014-2999(83)90393-x. [DOI] [PubMed] [Google Scholar]
- Butcher F. R., Putney J. W., Jr Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. [PubMed] [Google Scholar]
- Cohen R. A., Shepherd J. T., Vanhoutte P. M. Endothelium and asymmetrical responses of the coronary arterial wall. Am J Physiol. 1984 Sep;247(3 Pt 2):H403–H408. doi: 10.1152/ajpheart.1984.247.3.H403. [DOI] [PubMed] [Google Scholar]
- Conway R. S., Factor S. M., Sonnenblick E. H., Baez S. Microvascular reactivity of the myopathic Syrian hamster cremaster muscle. Cardiovasc Res. 1987 Nov;21(11):796–803. doi: 10.1093/cvr/21.11.796. [DOI] [PubMed] [Google Scholar]
- Cottrell J. E., Turndorf H. Intravenous nitroglycerin. Am Heart J. 1978 Oct;96(4):550–553. doi: 10.1016/0002-8703(78)90170-9. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982 Oct;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Diamond J., Chu E. B. Possible role for cyclic GMP in endothelium-dependent relaxation of rabbit aorta by acetylcholine. Comparison with nitroglycerin. Res Commun Chem Pathol Pharmacol. 1983 Sep;41(3):369–381. [PubMed] [Google Scholar]
- Franciosa J. A., Cohn J. N. Effects of minoxidil on hemodynamics in patients with congestive heart failure. Circulation. 1981 Mar;63(3):652–657. doi: 10.1161/01.cir.63.3.652. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gold H. K., Leinbach R. C., Sanders C. A. Use of sublingual nitroglycerin in congestive failure following acute myocardial infarction. Circulation. 1972 Nov;46(5):839–845. doi: 10.1161/01.cir.46.5.839. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Byrns R. E., Wood K. S., Chaudhuri G. Endothelium-derived relaxing factor and nitric oxide possess identical pharmacologic properties as relaxants of bovine arterial and venous smooth muscle. J Pharmacol Exp Ther. 1988 Jul;246(1):218–226. [PubMed] [Google Scholar]
- Ito T., Chiba S. Effects of prolonged cold storage on the responsiveness of isolated and perfused canine intermediate auricular artery. Arch Int Pharmacodyn Ther. 1985 May;275(1):13–21. [PubMed] [Google Scholar]
- Karliner J. S., Alabaster C., Stephens H., Barnes P., Dollery C. Enhanced noradrenaline response in cardiomyopathic hamsters: possible relation to changes in adrenoceptors studied by radioligand binding. Cardiovasc Res. 1981 May;15(5):296–304. doi: 10.1093/cvr/15.5.296. [DOI] [PubMed] [Google Scholar]
- Molina C. R., Andresen J. W., Rapoport R. M., Waldman S., Murad F. Effect of in vivo nitroglycerin therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J Cardiovasc Pharmacol. 1987 Oct;10(4):371–378. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- Parker R. B., Waud D. R. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. I. Agonists. J Pharmacol Exp Ther. 1971 Apr;177(1):1–12. [PubMed] [Google Scholar]
- Parmley W. W. Pathophysiology of congestive heart failure. Am J Cardiol. 1985 Jan 11;55(2):9A–14A. doi: 10.1016/0002-9149(85)90790-8. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Rubanyi G. M., McKinney M., Vanhoutte P. M. Biphasic release of endothelium-derived relaxing factor(s) by acetylcholine from perfused canine femoral arteries. Characterization of muscarinic receptors. J Pharmacol Exp Ther. 1987 Mar;240(3):802–808. [PubMed] [Google Scholar]
- Shirasaki Y., Su C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharmacol. 1985 Aug 7;114(1):93–96. doi: 10.1016/0014-2999(85)90527-8. [DOI] [PubMed] [Google Scholar]
- White D. G., Lewis M. J., Griffith T. M., Edwards D. H., Henderson A. H. Influence of endothelium on drug-induced relaxation of the rabbit aorta. Eur J Pharmacol. 1986 Feb 11;121(1):19–23. doi: 10.1016/0014-2999(86)90387-0. [DOI] [PubMed] [Google Scholar]



