Abstract
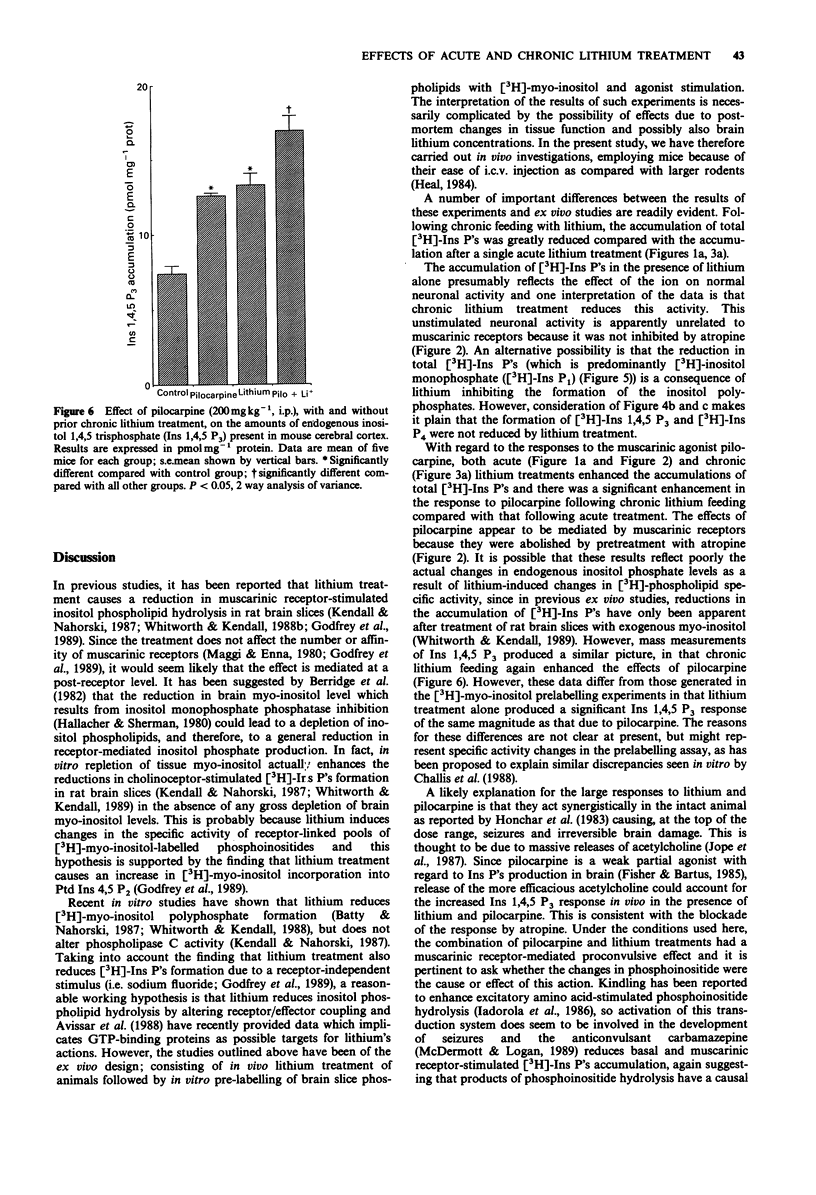
1. Measurements were made of the in vivo formation of inositol phosphates in the brains of C57/B1/601a mice treated acutely or chronically with lithium chloride (LiCl). 2. A single injection of LiCl (10 mEquiv kg-1, s.c.) 18 h before death increased the accumulation of [3H]-inositol phosphates ([3H]-Ins P's) in the brains of mice injected i.c.v. with [3H]-myo-inositol 24 h previously. 3. Pilocarpine (200 mg kg-1, i.p.) injected 15 min before death further enhanced the formation of [3H]-Ins P's in the brains of LiCl-treated, but not saline-treated, mice. The enhancement due to pilocarpine was abolished by injection of atropine sulphate (10 mg kg-1, i.p.) 10 min earlier. 4. Chronic (14 days) LiCl feeding produced an accumulation of [3H]-Ins P's significantly less than that due to a single injection of LiCl, but the response to pilocarpine was markedly greater in mice chronically fed with LiCl when compared with mice acutely injected with LiCl. 5. Mass measurements of endogenous inositol 1,4,5 triphosphate revealed increases due to pilocarpine and chronic LiCl feeding alone. A combination of the two treatments produced levels greater than either alone. 6. These results demonstrate that LiCl treatment enhances both basal and pilocarpine-stimulated inositol phospholipid hydrolysis in vivo and this might be relevant to its therapeutic effects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar S., Schreiber G., Danon A., Belmaker R. H. Lithium inhibits adrenergic and cholinergic increases in GTP binding in rat cortex. Nature. 1988 Feb 4;331(6155):440–442. doi: 10.1038/331440a0. [DOI] [PubMed] [Google Scholar]
- Batty I., Nahorski S. R. Lithium inhibits muscarinic-receptor-stimulated inositol tetrakisphosphate accumulation in rat cerebral cortex. Biochem J. 1987 Nov 1;247(3):797–800. doi: 10.1042/bj2470797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Batty I. H., Nahorski S. R. Mass measurements of inositol(1,4,5)trisphosphate in rat cerebral cortex slices using a radioreceptor assay: effects of neurotransmitters and depolarization. Biochem Biophys Res Commun. 1988 Dec 15;157(2):684–691. doi: 10.1016/s0006-291x(88)80304-8. [DOI] [PubMed] [Google Scholar]
- Ebstein R. P., Hermoni M., Belmaker R. H. The effect of lithium on noradrenaline-induced cyclic AMP accumulation in rat brain: inhibition after chronic treatment and absence of supersensitivity. J Pharmacol Exp Ther. 1980 Apr;213(1):161–167. [PubMed] [Google Scholar]
- Fisher S. K., Bartus R. T. Regional differences in the coupling of muscarinic receptors to inositol phospholipid hydrolysis in guinea pig brain. J Neurochem. 1985 Oct;45(4):1085–1095. doi: 10.1111/j.1471-4159.1985.tb05527.x. [DOI] [PubMed] [Google Scholar]
- Friedman E., Wang H. Y. Effect of chronic lithium treatment on 5-hydroxytryptamine autoreceptors and release of 5-[3H]hydroxytryptamine from rat brain cortical, hippocampal, and hypothalamic slices. J Neurochem. 1988 Jan;50(1):195–201. doi: 10.1111/j.1471-4159.1988.tb13249.x. [DOI] [PubMed] [Google Scholar]
- Godfrey P. P., McClue S. J., White A. M., Wood A. J., Grahame-Smith D. G. Subacute and chronic in vivo lithium treatment inhibits agonist- and sodium fluoride-stimulated inositol phosphate production in rat cortex. J Neurochem. 1989 Feb;52(2):498–506. doi: 10.1111/j.1471-4159.1989.tb09148.x. [DOI] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Heal D. J. Phenylephrine-induced activity in mice as a model of central alpha 1-adrenoceptor function. Effects of acute and repeated administration of antidepressant drugs and electroconvulsive shock. Neuropharmacology. 1984 Nov;23(11):1241–1251. doi: 10.1016/0028-3908(84)90040-6. [DOI] [PubMed] [Google Scholar]
- Honchar M. P., Olney J. W., Sherman W. R. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983 Apr 15;220(4594):323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- Iadorola M. J., Nicoletti F., Naranjo J. R., Putnam F., Costa E. Kindling enhances the stimulation of inositol phospholipid hydrolysis elicited by ibotenic acid in rat hippocampal slices. Brain Res. 1986 May 21;374(1):174–178. doi: 10.1016/0006-8993(86)90407-5. [DOI] [PubMed] [Google Scholar]
- Jope R. S., Simonato M., Lally K. Acetylcholine content in rat brain is elevated by status epilepticus induced by lithium and pilocarpine. J Neurochem. 1987 Sep;49(3):944–951. doi: 10.1111/j.1471-4159.1987.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Nahorski S. R. Acute and chronic lithium treatments influence agonist and depolarization-stimulated inositol phospholipid hydrolysis in rat cerebral cortex. J Pharmacol Exp Ther. 1987 Jun;241(3):1023–1027. [PubMed] [Google Scholar]
- Kennedy E. D., Batty I. H., Chilvers E. R., Nahorski S. R. A simple enzymic method to separate [3H]inositol 1,4,5- and 1,3,4-trisphosphate isomers in tissue extracts. Biochem J. 1989 May 15;260(1):283–286. doi: 10.1042/bj2600283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi A., Enna S. J. Regional alterations in rat brain neurotransmitter systems following chronic lithium treatment. J Neurochem. 1980 Apr;34(4):888–892. doi: 10.1111/j.1471-4159.1980.tb09662.x. [DOI] [PubMed] [Google Scholar]
- McDermott E. E., Logan S. D. Inhibition of agonist-stimulated inositol lipid metabolism by the anticonvulsant carbamazepine in rat hippocampus. Br J Pharmacol. 1989 Oct;98(2):581–589. doi: 10.1111/j.1476-5381.1989.tb12632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek J. L. Inositol bis-, tris-, and tetrakis(phosphate)s: analysis in tissues by HPLC. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4162–4166. doi: 10.1073/pnas.83.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup J. F., Friedel R. O., Shanberg S. M. Microwave irradiation fixation for studies of polyphosphoinositide metabolism in brain. J Neurochem. 1978 Mar;30(3):635–637. doi: 10.1111/j.1471-4159.1978.tb07819.x. [DOI] [PubMed] [Google Scholar]
- Turski W. A., Cavalheiro E. A., Bortolotto Z. A., Mello L. M., Schwarz M., Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984 Nov 12;321(2):237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Whitworth P., Kendall D. A. Effects of lithium on inositol phospholipid hydrolysis and inhibition of dopamine D1 receptor-mediated cyclic AMP formation by carbachol in rat brain slices. J Neurochem. 1989 Aug;53(2):536–541. doi: 10.1111/j.1471-4159.1989.tb07366.x. [DOI] [PubMed] [Google Scholar]
- Whitworth P., Kendall D. A. Lithium selectively inhibits muscarinic receptor-stimulated inositol tetrakisphosphate accumulation in mouse cerebral cortex slices. J Neurochem. 1988 Jul;51(1):258–265. doi: 10.1111/j.1471-4159.1988.tb04865.x. [DOI] [PubMed] [Google Scholar]
- Wood A. J., Goodwin G. M. A review of the biochemical and neuropharmacological actions of lithium. Psychol Med. 1987 Aug;17(3):579–600. doi: 10.1017/s0033291700025836. [DOI] [PubMed] [Google Scholar]


