Abstract
1. Bay K 8644 (0.33 nM to 1 microM) greatly increased the contractions of rat urinary bladder detrusor muscle induced by beta, gamma-methylene ATP (beta, gamma-MeATP, 10 microM) and by electrical field stimulation of the purinergic component (the cholinergic response was blocked by atropine). 2. The contractions induced by acetylcholine (ACh, 10 microM) and by electrical field stimulation of the cholinergic component (the purinergic response was blocked following desensitization by alpha, beta-MeATP) were also potentiated by Bay K 8644, although to a lesser extent than the purinergic responses. 3. Nifedipine (1 nM to 3.3 microM) inhibited all the contractions induced by beta, gamma-MeATP, ACh and electrical field stimulation. However, while the responses to beta, gamma-MeATP and electrical field stimulation of the purinergic component were almost abolished, a substantial proportion of the responses to ACh and electrical field stimulation of the cholinergic component were nifedipine resistant. 4. The concentration-effect curves for the potentiation by Bay K 8644 of the responses to beta, gamma-MeATP, ACh and electrical field stimulation were shifted to the right by nifedipine (10 nM). At concentrations greater than 1 microM, Bay K 8644 inhibited contraction. 5. It is concluded that voltage-sensitive calcium channels play an important role in the excitatory mechanical action of P2X-purinoceptor-mediated purinergic responses in the rat urinary bladder, while cholinergic-mediated responses are less dependent on such channels.
Full text
PDF
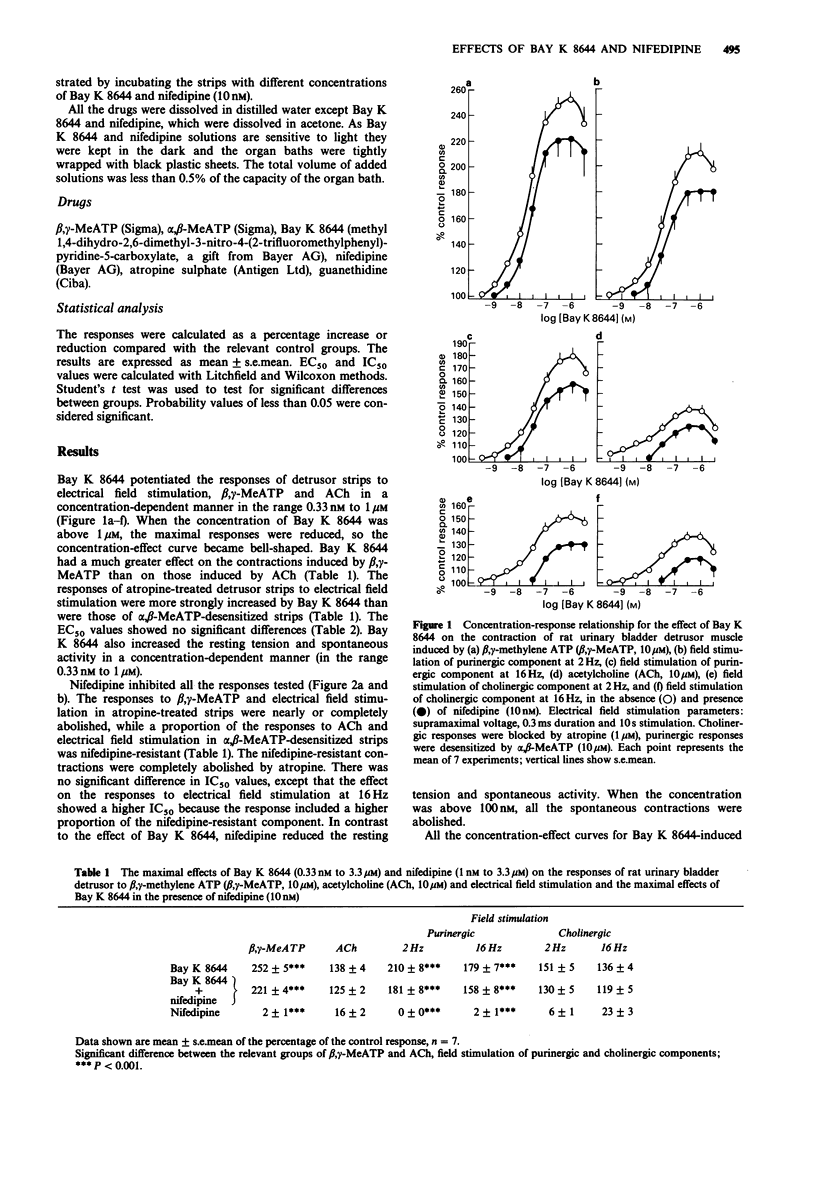
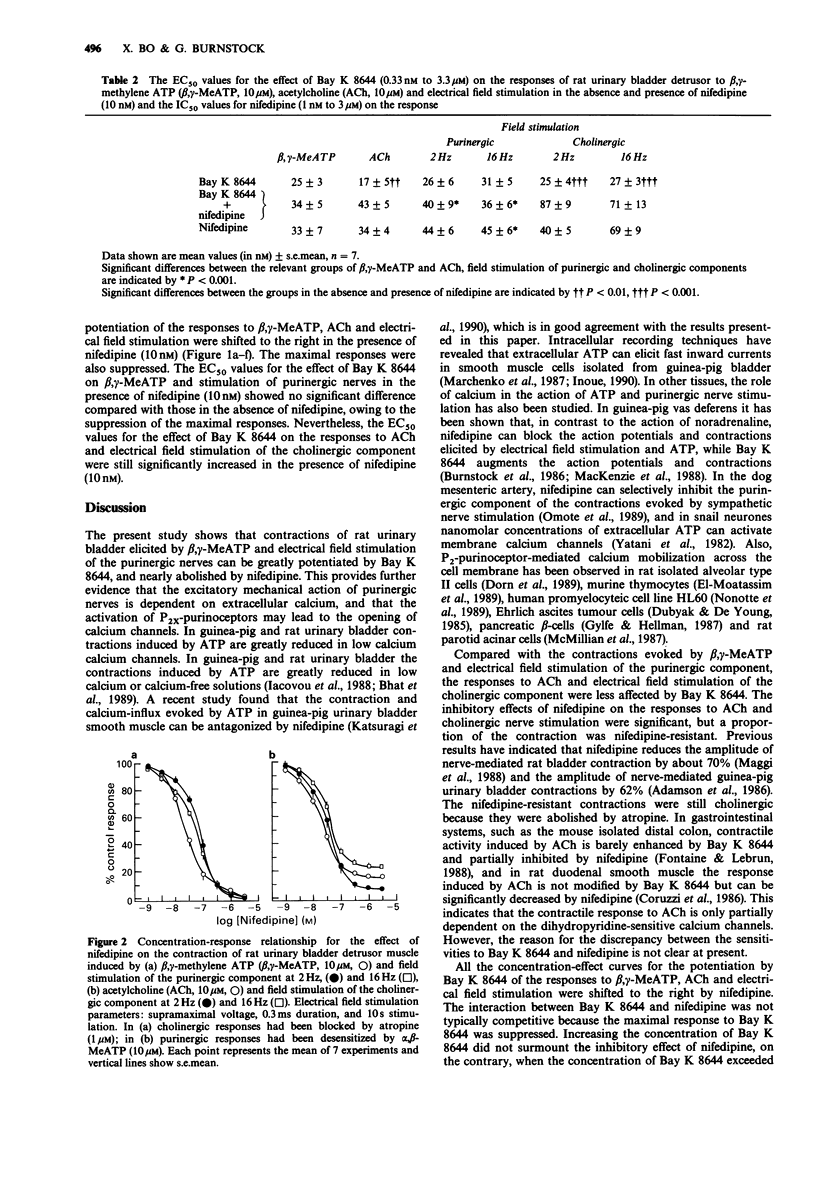


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechem M., Schramm M. Calcium-agonists. J Mol Cell Cardiol. 1987 May;19 (Suppl 2):63–75. doi: 10.1016/s0022-2828(87)80005-6. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bhat M. B., Mishra S. K., Raviprakash V. Differential susceptibility of cholinergic and noncholinergic neurogenic responses to calcium channel blockers and low Ca2+ medium in rat urinary bladder. Br J Pharmacol. 1989 Apr;96(4):837–842. doi: 10.1111/j.1476-5381.1989.tb11892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., Burnstock G., Cocks T. Effects of adenosine 5'-triphosphate (ATP) and beta-gamma-methylene ATP on the rat urinary bladder. Br J Pharmacol. 1979 Jan;65(1):97–102. doi: 10.1111/j.1476-5381.1979.tb17337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Burnstock G. The changing face of autonomic neurotransmission. Acta Physiol Scand. 1986 Jan;126(1):67–91. doi: 10.1111/j.1748-1716.1986.tb07790.x. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Poli E., Bertaccini G. Bay K 8644 and acetylcholine: different interaction with calcium ions in the rat duodenal muscle. Pharmacology. 1986;33(4):206–216. doi: 10.1159/000138218. [DOI] [PubMed] [Google Scholar]
- Dorn C. C., Rice W. R., Singleton F. M. Calcium mobilization and response recovery following P2-purinoceptor stimulation of rat isolated alveolar type II cells. Br J Pharmacol. 1989 May;97(1):163–170. doi: 10.1111/j.1476-5381.1989.tb11938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Fontaine J., Lebrun P. Pharmacological analysis of the effects of Bay K 8644 and organic calcium antagonists on the mouse isolated distal colon. Br J Pharmacol. 1988 Aug;94(4):1198–1205. doi: 10.1111/j.1476-5381.1988.tb11639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E., Hellman B. External ATP mimics carbachol in initiating calcium mobilization from pancreatic beta-cells conditioned by previous exposure to glucose. Br J Pharmacol. 1987 Oct;92(2):281–289. doi: 10.1111/j.1476-5381.1987.tb11322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourani S. M., Welford L. A., Cusack N. J. L-AMP-PCP, an ATP receptor agonist in guinea-pig bladder, is inactive on taenia coli. Eur J Pharmacol. 1985 Jan 22;108(2):197–200. doi: 10.1016/0014-2999(85)90726-5. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Katsuragi T., Usune S., Furukawa T. Antagonism by nifedipine of contraction and Ca2(+)-influx evoked by ATP in guinea-pig urinary bladder. Br J Pharmacol. 1990 Jun;100(2):370–374. doi: 10.1111/j.1476-5381.1990.tb15811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. A., Wakelam M. J. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983 Jun 16;303(5918):621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Smith T. W., Marsh J. D. Evidence for distinct calcium channel agonist and antagonist binding sites in intact cultured embryonic chick ventricular cells. Circ Res. 1987 May;60(5):683–691. doi: 10.1161/01.res.60.5.683. [DOI] [PubMed] [Google Scholar]
- MacKenzie I., Manzini S., Burnstock G. Regulation of voltage-dependent excitatory responses to alpha,beta-methylene ATP, ATP and non-adrenergic nerve stimulation by dihydropyridines in the guinea-pig vas deferens. Neuroscience. 1988 Oct;27(1):317–332. doi: 10.1016/0306-4522(88)90241-2. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Manzini S., Parlani M., Conte B., Giuliani S., Meli A. The effect of nifedipine on spontaneous, drug-induced and reflexly-activated contractions of the rat urinary bladder: evidence for the participation of an intracellular calcium store to micturition contraction. Gen Pharmacol. 1988;19(1):73–81. doi: 10.1016/0306-3623(88)90008-0. [DOI] [PubMed] [Google Scholar]
- Marchenko S. M., Volkova T. M., Fedorov O. I. ATF-aktiviruemaia ionnaia provodimost' v izolirovannykh gladkomyshechnykh kletkakh mochevogo puzyria morskoi svinki. Neirofiziologiia. 1987;19(1):95–100. [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Cantley L. C., Talamo B. R. Extracellular ATP elevates intracellular free calcium in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):523–530. doi: 10.1016/0006-291x(87)90399-8. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Adelstein R. S. Inhibition of turkey gizzard myosin light chain kinase activity by BAY K 8644. Eur J Pharmacol. 1984 Aug 3;103(1-2):161–163. doi: 10.1016/0014-2999(84)90204-8. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Omote S., Kigoshi S., Muramatsu I. Selective inhibition by nifedipine of the purinergic component of neurogenic vasoconstriction in the dog mesenteric artery. Eur J Pharmacol. 1989 Jan 31;160(2):239–245. doi: 10.1016/0014-2999(89)90496-2. [DOI] [PubMed] [Google Scholar]
- Thomas G., Gross R., Schramm M. Calcium channel modulation: ability to inhibit or promote calcium influx resides in the same dihydropyridine molecule. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1170–1176. [PubMed] [Google Scholar]
- White T. D. Role of adenine compounds in autonomic neurotransmission. Pharmacol Ther. 1988;38(2):129–168. doi: 10.1016/0163-7258(88)90095-2. [DOI] [PubMed] [Google Scholar]
- Yatani A., Tsuda Y., Akaike N., Brown A. M. Nanomolar concentrations of extracellular ATP activate membrane Ca channels in snail neurones. Nature. 1982 Mar 11;296(5853):169–171. doi: 10.1038/296169a0. [DOI] [PubMed] [Google Scholar]
- el-Moatassim C., Maurice T., Mani J. C., Dornand J. The [Ca2+]i increase induced in murine thymocytes by extracellular ATP does not involve ATP hydrolysis and is not related to phosphoinositide metabolism. FEBS Lett. 1989 Jan 2;242(2):391–396. doi: 10.1016/0014-5793(89)80508-3. [DOI] [PubMed] [Google Scholar]


