Abstract
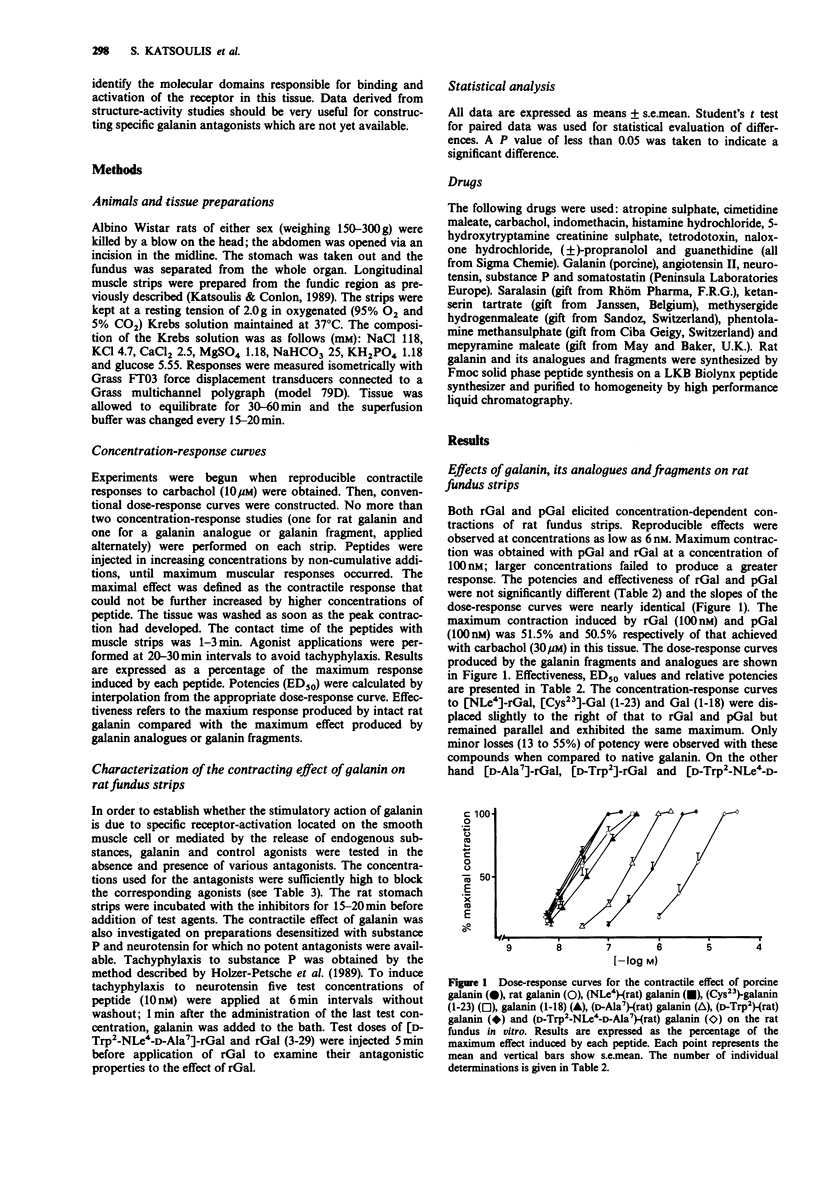
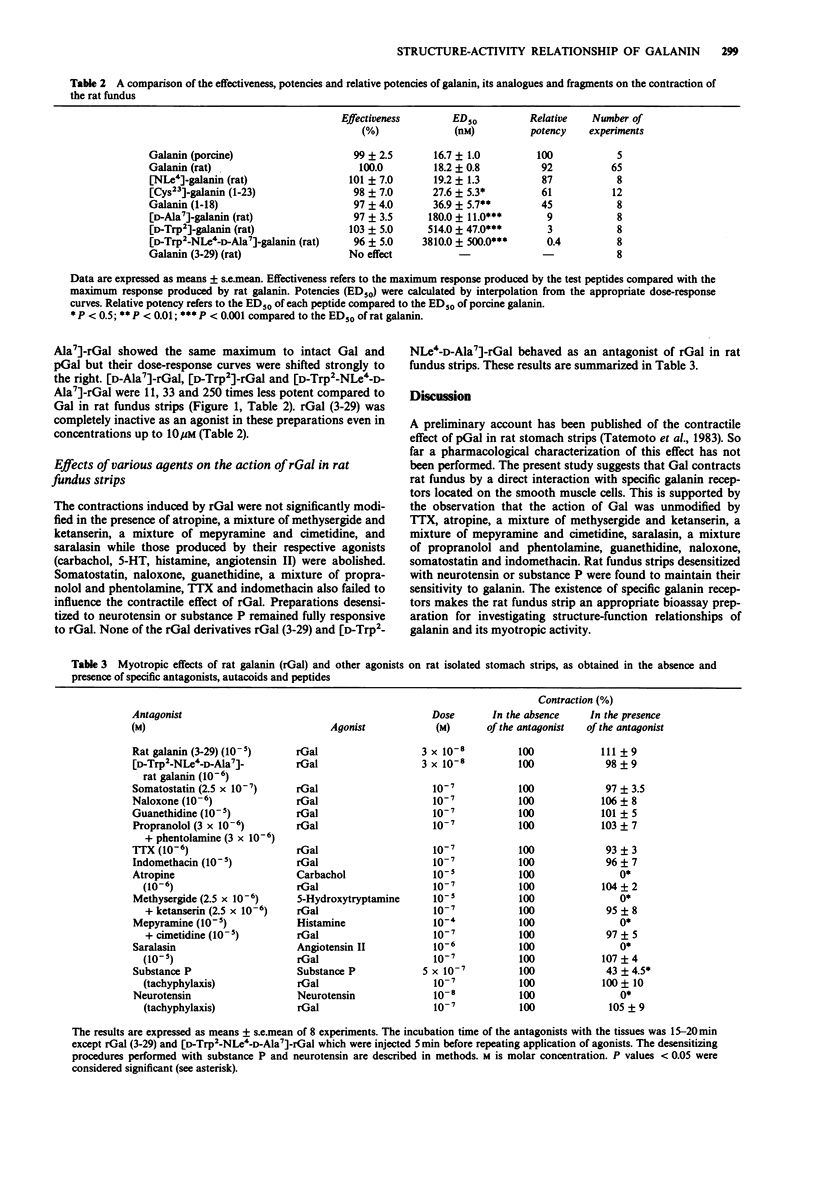
1. Rat and porcine galanin (rGal and pGal) produced dose-dependent contraction of rat fundus strips in a concentration range of 6 nM-100 nM. 2. The stimulatory effect of rGal on rat fundus strips was not modified in the presence of somatostatin (250 nM), naloxone (1 microM), guanethidine (10 microM), a mixture of propranolol (3 microM) and phentolamine (3 microM), tetrodotoxin (1 microM), indomethacin (10 microM), atropine (1 microM), a mixture of methysergide (2.5 microM) and ketanserine (2.5 microM), a mixture of mepyramine (10 microM) and cimetidine (10 microM), and saralasin (10 microM) or when strips were desensitized to substance P and neurotensin. 3. These results suggest the localization of specific Gal receptors on the surface of smooth muscle cells of rat fundus. 4. The galanin analogues [D-Trp2]-rGal, [Nle4]-rGal, [D-Ala7]-rGal, [D-Trp2-NLe4-D-Ala7]-rGal and fragments [Cys23]-Gal (1-23), Gal (1-18) were fully active. In contrast, rGal (3-29) was completely inactive and showed no antagonistic properties to the contractile effect of intact galanin. 5. The order of potency of the galanin peptides, analogues and fragments to contract rat fundus strips was: pGal greater than rGal greater than [NLe4]-rGal greater than [Cys23]-Gal (1-23) greater than Gal (1-18) greater than [D-Ala7]-rGal greater than [Trp2]-rGal greater than [D-Trp2-NLe4-D-Ala7]-rGal. 6. The data originating from our structure-activity study suggest that the C-terminal portion of Gal contributes mainly to the affinity of Gal receptors whereas the N-terminal portion of Gal is responsible for the full activation of Gal receptors in this tissue.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiranoff B., Lorinet A. M., Yanaihara N., Laburthe M. Structural requirement for galanin action in the pancreatic beta cell line Rin m 5F. Eur J Pharmacol. 1989 Apr 12;163(1):205–207. doi: 10.1016/0014-2999(89)90421-4. [DOI] [PubMed] [Google Scholar]
- Bauer F. E., Adrian T. E., Christofides N. D., Ferri G. L., Yanaihara N., Polak J. M., Bloom S. R. Distribution and molecular heterogeneity of galanin in human, pig, guinea pig, and rat gastrointestinal tracts. Gastroenterology. 1986 Oct;91(4):877–883. doi: 10.1016/0016-5085(86)90689-x. [DOI] [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Bauer F. E., Christofides N. D., Carlei F., Bloom S. R. Occurrence and distribution of a newly discovered peptide, galanin, in the mammalian enteric nervous system. Gut. 1986 Jul;27(7):849–857. doi: 10.1136/gut.27.7.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Arnbjörnsson E., Ekman R., Håkanson R., Sundler F. Neuropeptides in the human appendix. Distribution and motor effects. Dig Dis Sci. 1989 Aug;34(8):1217–1230. doi: 10.1007/BF01537270. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Håkanson R., Sundler F., Wahlestedt C. Galanin: neuromodulatory and direct contractile effects on smooth muscle preparations. Br J Pharmacol. 1985 Sep;86(1):241–246. doi: 10.1111/j.1476-5381.1985.tb09455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Rökaeus A., Håkanson R., Sundler F. Galanin nerve fibers in the rat gut: distribution, origin and projections. Neuroscience. 1985 Oct;16(2):355–363. doi: 10.1016/0306-4522(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Fontaine J., Lebrun P. Galanin: Ca2+-dependent contractile effects on the isolated mouse distal colon. Eur J Pharmacol. 1989 May 30;164(3):583–586. doi: 10.1016/0014-2999(89)90268-9. [DOI] [PubMed] [Google Scholar]
- Fox J. E., McDonald T. J., Kostolanska F., Tatemoto K. Galanin: an inhibitory neural peptide of the canine small intestine. Life Sci. 1986 Jul 14;39(2):103–110. doi: 10.1016/0024-3205(86)90443-1. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Rökaeus A., McDonald T. J., Brooks B. Galanin-immunoreactive neurons in the guinea-pig small intestine: their projections and relationships to other enteric neurons. Cell Tissue Res. 1987 Dec;250(3):607–615. doi: 10.1007/BF00218954. [DOI] [PubMed] [Google Scholar]
- Holzer-Petsche U., Seitz H., Lembeck F. Effect of capsaicin on gastric corpus smooth muscle of the rat in vitro. Eur J Pharmacol. 1989 Mar 14;162(1):29–36. doi: 10.1016/0014-2999(89)90600-6. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Spindel E. R., Isselbacher K. J., Chin W. W. Tissue-specific expression of the rat galanin gene. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1065–1069. doi: 10.1073/pnas.85.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsoulis S., Conlon J. M. Calcitonin gene-related peptides relax guinea pig and rat gastric smooth muscle. Eur J Pharmacol. 1989 Mar 14;162(1):129–134. doi: 10.1016/0014-2999(89)90612-2. [DOI] [PubMed] [Google Scholar]
- Kuwahara A., Ozaki T., Yanaihara N. Galanin suppresses neurally evoked contractions of circular muscle in the guinea-pig ileum. Eur J Pharmacol. 1989 May 2;164(1):175–178. doi: 10.1016/0014-2999(89)90247-1. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Santicioli P., Giuliani S., Turini D., Barbanti G., Beneforti P., Misuri D., Meli A. Human isolated small intestine: motor responses of the longitudinal muscle to field stimulation and exogenous neuropeptides. Naunyn Schmiedebergs Arch Pharmacol. 1989 Apr;339(4):415–423. doi: 10.1007/BF00736056. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Patacchini R., Turini D., Barbanti G., Beneforti P., Giuliani S., Meli A. Galanin: a potent modulator of excitatory neurotransmission in the human urinary bladder. Eur J Pharmacol. 1987 Nov 3;143(1):135–137. doi: 10.1016/0014-2999(87)90744-8. [DOI] [PubMed] [Google Scholar]
- Melander T., Hökfelt T., Rökaeus A., Fahrenkrug J., Tatemoto K., Mutt V. Distribution of galanin-like immunoreactivity in the gastro-intestinal tract of several mammalian species. Cell Tissue Res. 1985;239(2):253–270. doi: 10.1007/BF00218003. [DOI] [PubMed] [Google Scholar]
- Ohhashi T., Jacobowitz D. M. Galanin potentiates electrical stimulation and exogenous norepinephrine-induced contractions in the rat vas deferens. Regul Pept. 1985 Oct;12(2):163–171. doi: 10.1016/0167-0115(85)90197-1. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Schemann M., Tamura K., Wood J. D. Galanin mimics slow synaptic inhibition in myenteric neurons. Eur J Pharmacol. 1986 May 27;124(3):379–380. doi: 10.1016/0014-2999(86)90246-3. [DOI] [PubMed] [Google Scholar]
- Rökaeus A., Melander T., Hökfelt T., Lundberg J. M., Tatemoto K., Carlquist M., Mutt V. A galanin-like peptide in the central nervous system and intestine of the rat. Neurosci Lett. 1984 Jun 15;47(2):161–166. doi: 10.1016/0304-3940(84)90423-3. [DOI] [PubMed] [Google Scholar]
- Tamura K., Palmer J. M., Winkelmann C. K., Wood J. D. Mechanism of action of galanin on myenteric neurons. J Neurophysiol. 1988 Sep;60(3):966–979. doi: 10.1152/jn.1988.60.3.966. [DOI] [PubMed] [Google Scholar]
- Tamura K., Palmer J. M., Wood J. D. Galanin suppresses nicotinic synaptic transmission in the myenteric plexus of guinea-pig small intestine. Eur J Pharmacol. 1987 Apr 29;136(3):445–446. doi: 10.1016/0014-2999(87)90323-2. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Rökaeus A., Jörnvall H., McDonald T. J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983 Nov 28;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- Vrontakis M. E., Peden L. M., Duckworth M. L., Friesen H. G. Isolation and characterization of a complementary DNA (galanin) clone from estrogen-induced pituitary tumor messenger RNA. J Biol Chem. 1987 Dec 15;262(35):16755–16758. [PubMed] [Google Scholar]
- Yau W. M., Dorsett J. A., Youther M. L. Evidence for galanin as an inhibitory neuropeptide on myenteric cholinergic neurons in the guinea pig small intestine. Neurosci Lett. 1986 Dec 23;72(3):305–308. doi: 10.1016/0304-3940(86)90531-8. [DOI] [PubMed] [Google Scholar]


