Abstract
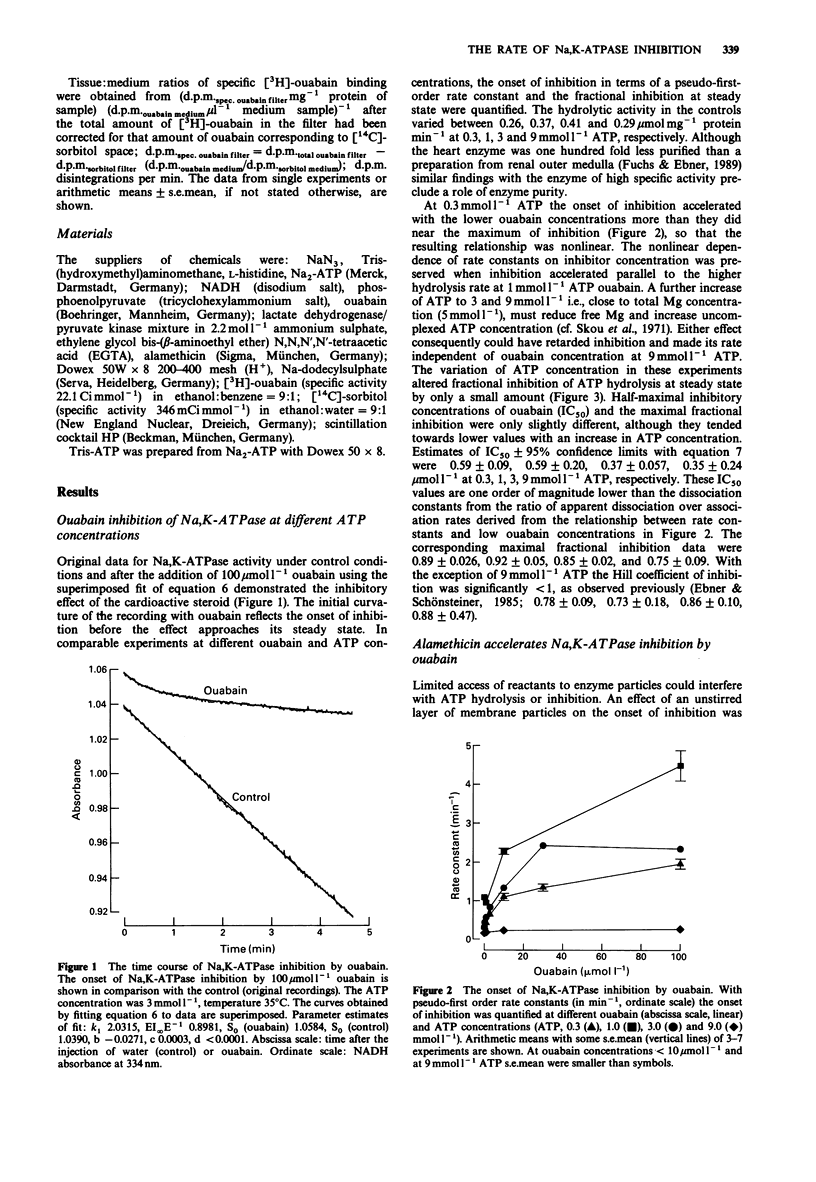
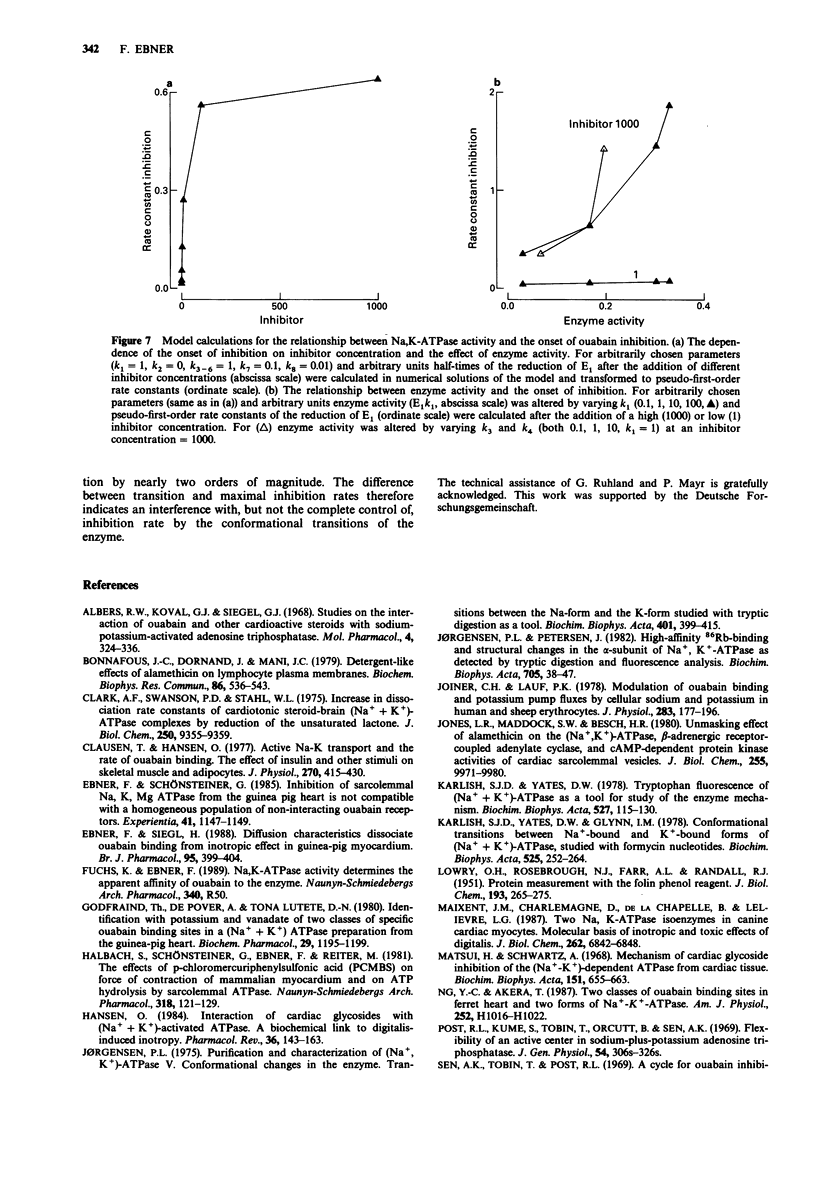
1. The onset of ouabain inhibition was quantified by analysis with an integrated rate equation from experiments in which the activity of Na,K-ATPase from guinea-pig myocardium had been altered with adenosine 5'-triphosphate (ATP, 0.3-9 mmoll-1) in the absence and presence of a detergent. 2. Under control conditions with increasing ouabain (0.1-100 mumoll-1) and ATP (0.3-1 mmoll-1) concentrations, inhibition developed faster. The acceleration by ouabain became less effective at saturating concentrations leading to a non-linear relationship between pseudo-first-order rate constants of inhibition and ouabain concentration. With a rise of ATP to 3 and 9 mmoll-1, i.e., near total Mg concentration (5 mmoll-1), inhibition was retarded presumably because the free concentrations of Mg and uncomplexed ATP changed. Varying the ATP concentration had little effect on ouabain potency at steady state; Hill coefficients were less than 1. 3. The detergent alamethicin (23 micrograms ml-1) neither interfered with Na,K-ATPase activity nor with inhibition at steady state but accelerated its onset. This supports a role for a lipid barrier in the development of inhibition. 4. While the reaction of low concentrations of ouabain with the receptors seemed to govern inhibition rate, with an increase in steroid concentration in the presence of alamethicin, ATP-dependent enzyme activity interfered with the onset of inhibition. The transition of the enzyme between ouabain-sensitive and ATP-hydrolytic conformations consequently causes the non-linear concentration-dependence of pseudo-first-order rate constants. As the Hill coefficient was less than 1, a reaction of ouabain with two receptors also could have contributed to the special concentration-dependence of inhibition rates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers R. W., Koval G. J., Siegel Studies on the interaction of ouabain and other cardio-active steroids with sodium-potassium-activated adenosine triphosphatase. Mol Pharmacol. 1968 Jul;4(4):324–336. [PubMed] [Google Scholar]
- Bonnafous J. C., Dornand J., Mani J. C. Detergent-like effects of alamethicin on lymphocyte plasma membranes. Biochem Biophys Res Commun. 1979 Feb 14;86(3):536–544. doi: 10.1016/0006-291x(79)91747-9. [DOI] [PubMed] [Google Scholar]
- Clark A. F., Swanson P. D., Stahl W. L. Increase in dissociation rate constants of cardiotonic steroid-brain (Na+ + K+)-ATPase complexes by reduction of the unsaturated lactone. J Biol Chem. 1975 Dec 25;250(24):9355–9359. [PubMed] [Google Scholar]
- Clausen T., Hansen O. Active Na-K transport and the rate of ouabain binding. The effect of insulin and other stimuli on skeletal muscle and adipocytes. J Physiol. 1977 Sep;270(2):415–430. doi: 10.1113/jphysiol.1977.sp011959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F., Schönsteiner G. Inhibition of sarcolemmal Na, K, Mg ATPase from the guinea pig heart is not compatible with a homogeneous population of non-interacting ouabain receptors. Experientia. 1985 Sep 15;41(9):1147–1149. doi: 10.1007/BF01951701. [DOI] [PubMed] [Google Scholar]
- Ebner F., Siegl H. Diffusion characteristics dissociate ouabain binding from inotropic effect in guinea-pig myocardium. Br J Pharmacol. 1988 Oct;95(2):399–404. doi: 10.1111/j.1476-5381.1988.tb11659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T., De Pover A., Lutete D. N. Identification with potassium and vanadate of two classes of specific ouabain binding sites in a (Na+ + K+) ATPase preparation from the guinea-pig heart. Biochem Pharmacol. 1980 Apr 15;29(8):1195–1199. doi: 10.1016/0006-2952(80)90418-9. [DOI] [PubMed] [Google Scholar]
- Halbach S., Schönsteiner G., Ebner F., Reiter M. The effects of p-chloromercuriphenylsulfonic acid (PCMBS) on force of contraction of mammalian myocardium and on ATP hydrolysis by sarcolemmal ATPase. Naunyn Schmiedebergs Arch Pharmacol. 1981 Dec;318(2):121–129. doi: 10.1007/BF00508836. [DOI] [PubMed] [Google Scholar]
- Hansen O. Interaction of cardiac glycosides with (Na+ + K+)-activated ATPase. A biochemical link to digitalis-induced inotropy. Pharmacol Rev. 1984 Sep;36(3):143–163. [PubMed] [Google Scholar]
- Joiner C. H., Lauf P. K. Modulation of ouabain binding and potassium pump fluxes by cellular sodium and potassium in human and sheep erythrocytes. J Physiol. 1978 Oct;283:177–196. doi: 10.1113/jphysiol.1978.sp012495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. R., Maddock S. W., Besch H. R., Jr Unmasking effect of alamethicin on the (Na+,K+)-ATPase, beta-adrenergic receptor-coupled adenylate cyclase, and cAMP-dependent protein kinase activities of cardiac sarcolemmal vesicles. J Biol Chem. 1980 Oct 25;255(20):9971–9980. [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+, K+)-ATPase. V. Conformational changes in the enzyme Transitions between the Na-form and the K-form studied with tryptic digestion as a tool. Biochim Biophys Acta. 1975 Sep 2;401(3):399–415. doi: 10.1016/0005-2736(75)90239-4. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Petersen J. High-affinity 86Rb-binding and structural changes in the alpha-subunit of Na+,K+-ATPase as detected by tryptic digestion and fluorescence analysis. Biochim Biophys Acta. 1982 Jul 12;705(1):38–47. doi: 10.1016/0167-4838(82)90333-8. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W., Glynn I. M. Conformational transitions between Na+-bound and K+-bound forms of (Na+ + K+)-ATPase, studied with formycin nucleotides. Biochim Biophys Acta. 1978 Jul 7;525(1):252–264. doi: 10.1016/0005-2744(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W. Tryptophan fluorescence of (Na+ + K+)-ATPase as a tool for study of the enzyme mechanism. Biochim Biophys Acta. 1978 Nov 10;527(1):115–130. doi: 10.1016/0005-2744(78)90261-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maixent J. M., Charlemagne D., de la Chapelle B., Lelievre L. G. Two Na,K-ATPase isoenzymes in canine cardiac myocytes. Molecular basis of inotropic and toxic effects of digitalis. J Biol Chem. 1987 May 15;262(14):6842–6848. [PubMed] [Google Scholar]
- Matsui H., Schwartz A. Mechanism of cardiac glycoside inhibition of the (Na+-K+)-dependent ATPase from cardiac tissue. Biochim Biophys Acta. 1968 Mar 25;151(3):655–663. doi: 10.1016/0005-2744(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Ng Y. C., Akera T. Two classes of ouabain binding sites in ferret heart and two forms of Na+-K+-ATPase. Am J Physiol. 1987 May;252(5 Pt 2):H1016–H1022. doi: 10.1152/ajpheart.1987.252.5.H1016. [DOI] [PubMed] [Google Scholar]
- Sen A. K., Tobin T. A cycle for ouabain inhibition of sodium- and potassium-dependent adenosine triphosphatase. J Biol Chem. 1969 Dec 25;244(24):6596–6604. [PubMed] [Google Scholar]
- Skou J. C., Butler K. W., Hansen O. The effect of magnesium, ATP, P i , and sodium on the inhibition of the (Na + + K + )-activated enzyme system by g-strophanthin. Biochim Biophys Acta. 1971 Aug 13;241(2):443–461. doi: 10.1016/0005-2736(71)90044-7. [DOI] [PubMed] [Google Scholar]
- Skou J. C. Effects of ATP on the intermediary steps of the reaction of the (Na+ + K+)-ATPase. IV. Effect of ATP on K0.5 for Na+ and on hydrolysis at different pH and temperature. Biochim Biophys Acta. 1979 Apr 12;567(2):421–435. doi: 10.1016/0005-2744(79)90128-1. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Effects of ATP and protons on the Na : K selectivity of the (Na+ + K+)-ATPase studied by ligand effects on intrinsic and extrinsic fluorescence. Biochim Biophys Acta. 1980 Sep 18;601(2):386–402. doi: 10.1016/0005-2736(80)90543-x. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. The effects of Na+ and K+ on the conformational transitions of (Na+ + K+)-ATPase. Biochim Biophys Acta. 1983 Jul 28;746(1-2):101–113. doi: 10.1016/0167-4838(83)90016-x. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989 May 9;988(2):185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Two molecular forms of (Na+ + K+)-stimulated ATPase in brain. Separation, and difference in affinity for strophanthidin. J Biol Chem. 1979 Jul 10;254(13):6060–6067. [PubMed] [Google Scholar]
- Taniguchi K., Iida S. The role of phospholipids in the binding of ouabain to sodium- and potassium-dependent adenosine triphosphatase. Mol Pharmacol. 1973 May;9(3):350–359. [PubMed] [Google Scholar]
- Wallick E. T., Schwartz A. Thermodynamics of the rate of binding of oubain to the sodium, potassium-adenosine triphosphatase. J Biol Chem. 1974 Aug 25;249(16):5141–5147. [PubMed] [Google Scholar]
- Yoda A. Structue-activity relationships of cardiotonic steroids for the inhibition of sodium- and potassium-dependent adenosine triphosphatase. I. Dissociation rate constants of various enzyme-cardiac glycoside complexes formed in the presence of magnesium and phosphate. Mol Pharmacol. 1973 Jan;9(1):51–60. [PubMed] [Google Scholar]
- Yoda A., Yoda S., Sarrif A. M. Structure-activity relationships of cardiotonic steroids for the inhibition of sodium- and potassium-dependent adenosine triphosphatase. II. Association rate constants of various enzyme-cardiac glycoside complexes. Mol Pharmacol. 1973 Nov;9(6):766–773. [PubMed] [Google Scholar]
- Yoda A., Yoda S. Structure-activity relationships of cardiotonic steroids for the inhibition of sodium- and potassium-dependent adenosine triphosphatase. 3. Dissociation rate constants of various enzyme-cardiac glycoside complexes formed in the presence of sodium, magnesium, and adenosine triphosphate. Mol Pharmacol. 1974 May;10(3):494–500. [PubMed] [Google Scholar]


