Abstract
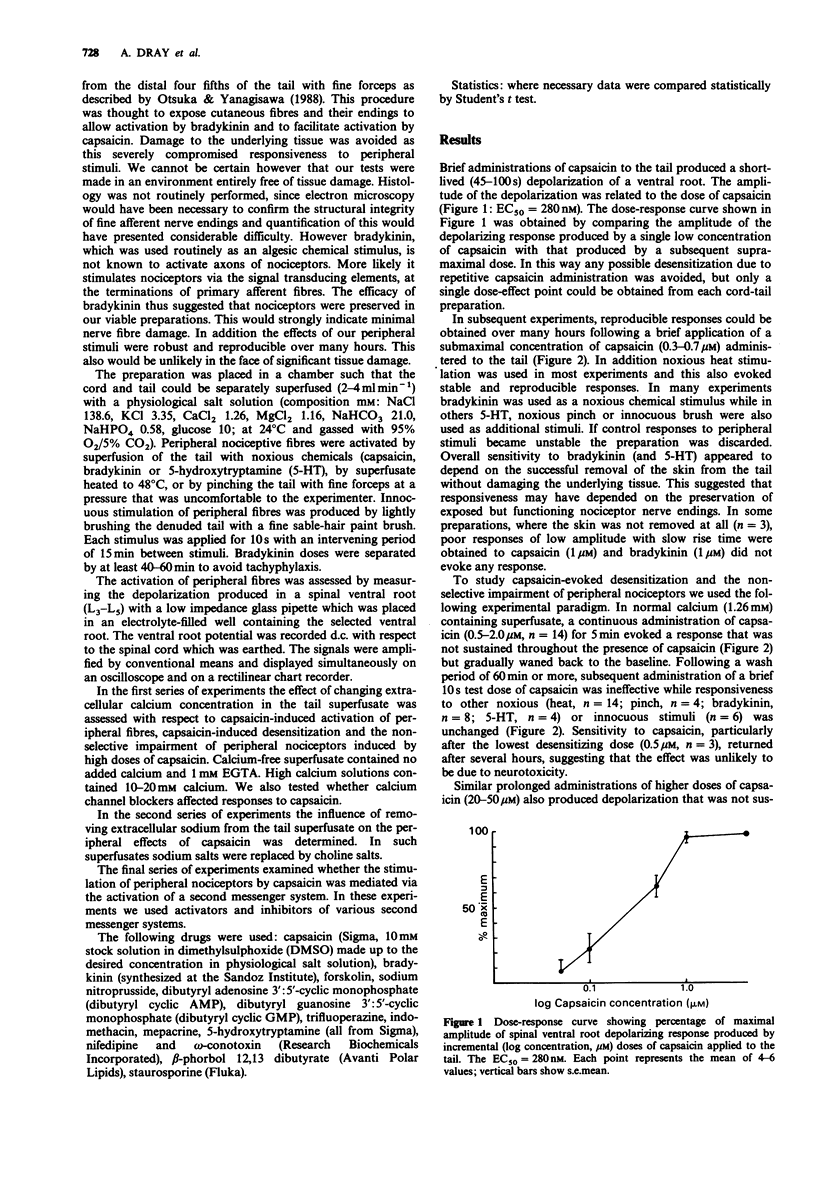
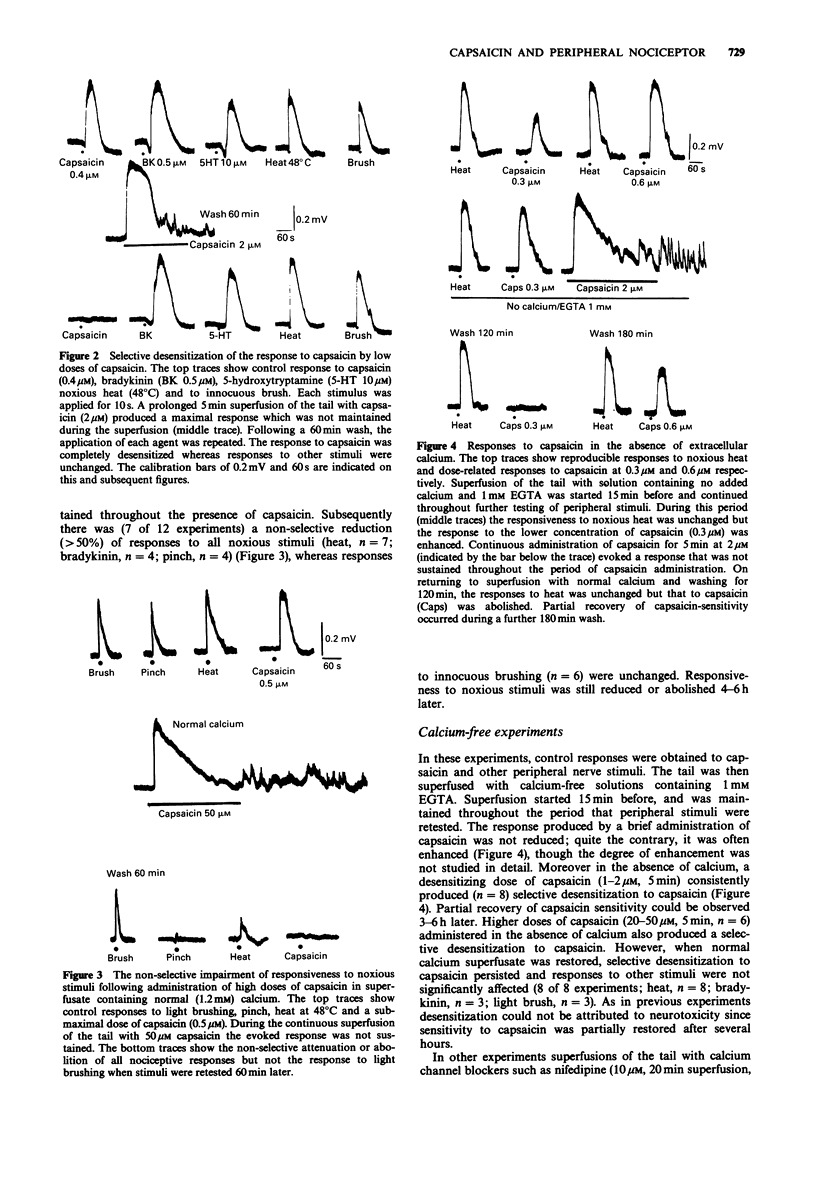
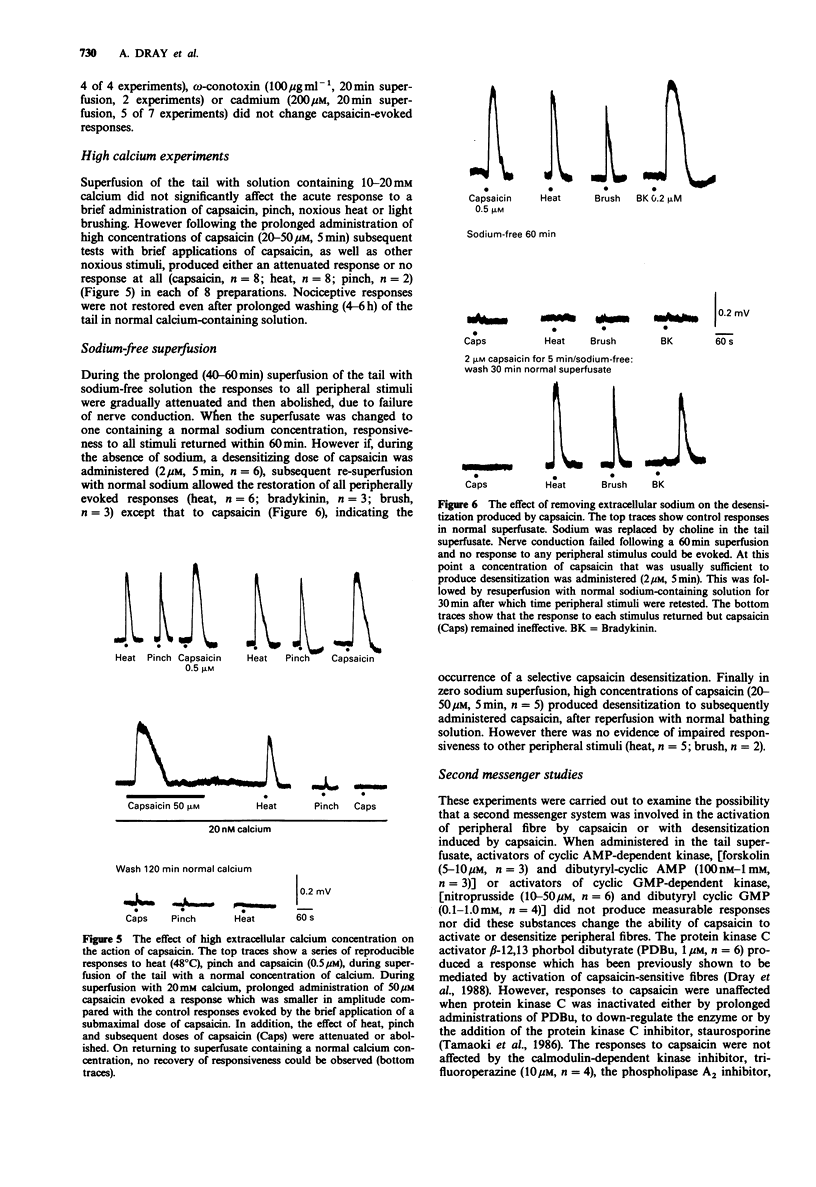
1. We have tested the hypothesis that capsaicin-induced activation, desensitization and impairment of peripheral nociceptor function is mediated by separate mechanisms. This was investigated by use of an in vitro preparation of the neonatal rat spinal cord with the functionally attached tail in which the cord and tail were separately superfused with physiological solution. Activation of peripheral fibres by noxious (capsaicin, bradykinin, 5-hydroxytrptamine, heat, pinch) and innocuous (light brush) stimuli was assessed by recording the depolarization of a spinal ventral root (L3-L5). 2. Brief administration of capsaicin produced dose-related depolarizing responses (EC50 = 280 nM). These responses could be reproduced for many hours following the repeated application of capsaicin at a submaximal concentration. Prolonged application of 0.5-2.0 microM capsaicin induced a selective desensitization to subsequent brief administrations of capsaicin. Prolonged administration at 20-50 microM produced an additional non-selective reduction in responses to all noxious stimuli without changing innocuous brush responses. 3. Removal of extracellular calcium from the tail superfusate did not reduce the response to capsaicin or prevent capsaicin-induced desensitization. However, high concentrations of capsaicin no longer induced a non-specific reduction of responses to other noxious stimuli. The response to a brief administration of capsaicin was unaffected by calcium channel blocking drugs including nifedipine, cadmium or omega-conotoxin. On the other hand high extracellular calcium increased the incidence of the non-selective reduction of responses to all noxious stimuli produced by high concentrations of capsaicin. 4. Replacement of extracellular sodium with choline blocked peripheral nerve conduction but did not prevent the desensitization produced by capsaicin.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi H., Konishi S., Otsuka M., Yanagisawa M. The role of substance P as a neurotransmitter in the reflexes of slow time courses in the neonatal rat spinal cord. Br J Pharmacol. 1985 Mar;84(3):663–673. doi: 10.1111/j.1476-5381.1985.tb16148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini P. I., Hogan P. G. Some rat sensory neurons in culture express characteristics of differentiated pain sensory cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):594–598. doi: 10.1073/pnas.80.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski R., Lynn B., Pini A. The effects of locally applied capsaicin on conduction in cutaneous nerves in four mammalian species. Br J Pharmacol. 1986 Oct;89(2):267–276. doi: 10.1111/j.1476-5381.1986.tb10256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Cachelin A. B., Colquhoun D. Desensitization of the acetylcholine receptor of frog end-plates measured in a Vaseline-gap voltage clamp. J Physiol. 1989 Aug;415:159–188. doi: 10.1113/jphysiol.1989.sp017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Bettaney J., Forster P. Capsaicin desensitization of peripheral nociceptive fibres does not impair sensitivity to other noxious stimuli. Neurosci Lett. 1989 Apr 24;99(1-2):50–54. doi: 10.1016/0304-3940(89)90263-2. [DOI] [PubMed] [Google Scholar]
- Dray A., Bettaney J., Forster P., Perkins M. N. Bradykinin-induced stimulation of afferent fibres is mediated through protein kinase C. Neurosci Lett. 1988 Sep 12;91(3):301–307. doi: 10.1016/0304-3940(88)90697-0. [DOI] [PubMed] [Google Scholar]
- Dray A., Bettaney J., Forster P. Resiniferatoxin, a potent capsaicin-like stimulator of peripheral nociceptors in the neonatal rat tail in vitro. Br J Pharmacol. 1990 Feb;99(2):323–326. doi: 10.1111/j.1476-5381.1990.tb14702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. Capsaicin and sensory neurones--a review. Pain. 1983 Feb;15(2):109–130. doi: 10.1016/0304-3959(83)90012-x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. J Physiol. 1987 Feb;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J Physiol. 1985 Jul;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. G., Hawcock A. B., Hill R. G. The depolarising action of capsaicin on rat isolated sciatic nerve. Life Sci. 1984 Oct 8;35(15):1561–1568. doi: 10.1016/0024-3205(84)90354-0. [DOI] [PubMed] [Google Scholar]
- Hayes A. G., Oxford A., Reynolds M., Shingler A. H., Skingle M., Smith C., Tyers M. B. The effects of a series of capsaicin analogues on nociception and body temperature in the rat. Life Sci. 1984 Mar 26;34(13):1241–1248. doi: 10.1016/0024-3205(84)90546-0. [DOI] [PubMed] [Google Scholar]
- Hayes A. G., Tyers M. B. Effects of capsaicin on nociceptive heat, pressure and chemical thresholds and on substance P levels in the rat. Brain Res. 1980 May 12;189(2):561–564. doi: 10.1016/0006-8993(80)90369-8. [DOI] [PubMed] [Google Scholar]
- Heyman I., Rang H. P. Depolarizing responses to capsaicin in a subpopulation of rat dorsal root ganglion cells. Neurosci Lett. 1985 May 1;56(1):69–75. doi: 10.1016/0304-3940(85)90442-2. [DOI] [PubMed] [Google Scholar]
- James I. F., Walpole C. S., Hixon J., Wood J. N., Wrigglesworth R. Long-lasting agonist activity produced by a capsaicin-like photoaffinity probe. Mol Pharmacol. 1988 Jun;33(6):643–649. [PubMed] [Google Scholar]
- Jancsó G., Király E., Joó F., Such G., Nagy A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurons in the adult rat. Neurosci Lett. 1985 Aug 30;59(2):209–214. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- Marsh S. J., Stansfeld C. E., Brown D. A., Davey R., McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987 Oct;23(1):275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Yanagisawa M. Effect of a tachykinin antagonist on a nociceptive reflex in the isolated spinal cord-tail preparation of the newborn rat. J Physiol. 1988 Jan;395:255–270. doi: 10.1113/jphysiol.1988.sp016917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsche U., Fleischer E., Lembeck F., Handwerker H. O. The effect of capsaicin application to a peripheral nerve on impulse conduction in functionally identified afferent nerve fibres. Brain Res. 1983 Apr 18;265(2):233–240. doi: 10.1016/0006-8993(83)90337-2. [DOI] [PubMed] [Google Scholar]
- Schäfer K. A quantitative study of the dependence of feline cold receptor activity on the calcium concentration. Pflugers Arch. 1987 Jun;409(1-2):208–213. doi: 10.1007/BF00584773. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Szallasi A., Blumberg P. M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30(2):515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J., Jancsó-Gábor A. Sensory effects of capsaicin congeners I. Relationship between chemical structure and pain-producing potency of pungent agents. Arzneimittelforschung. 1975;25(12):1877–1881. [PubMed] [Google Scholar]
- Szolcsányi J., Jancsó-Gábor A. Sensory effects of capsaicin congeners. Part II: Importance of chemical structure and pungency in desensitizing activity of capsaicin-type compounds. Arzneimittelforschung. 1976;26(1):33–37. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Waddell P. J., Lawson S. N. The C-fibre conduction block caused by capsaicin on rat vagus nerve in vitro. Pain. 1989 Nov;39(2):237–242. doi: 10.1016/0304-3959(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Winter J. Characterization of capsaicin-sensitive neurones in adult rat dorsal root ganglion cultures. Neurosci Lett. 1987 Sep 23;80(2):134–140. doi: 10.1016/0304-3940(87)90642-2. [DOI] [PubMed] [Google Scholar]
- Winter J., Dray A., Wood J. N., Yeats J. C., Bevan S. Cellular mechanism of action of resiniferatoxin: a potent sensory neuron excitotoxin. Brain Res. 1990 Jun 18;520(1-2):131–140. doi: 10.1016/0006-8993(90)91698-g. [DOI] [PubMed] [Google Scholar]
- Winter J., Forbes C. A., Sternberg J., Lindsay R. M. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988 Dec;1(10):973–981. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Coote P. R., Minhas A., Mullaney I., McNeill M., Burgess G. M. Capsaicin-induced ion fluxes increase cyclic GMP but not cyclic AMP levels in rat sensory neurones in culture. J Neurochem. 1989 Oct;53(4):1203–1211. doi: 10.1111/j.1471-4159.1989.tb07416.x. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Winter J., James I. F., Rang H. P., Yeats J., Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988 Sep;8(9):3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Murakoshi T., Tamai S., Otsuka M. Tail-pinch method in vitro and the effects of some antinociceptive compounds. Eur J Pharmacol. 1984 Nov 13;106(2):231–239. doi: 10.1016/0014-2999(84)90710-6. [DOI] [PubMed] [Google Scholar]


