Abstract
1. The structural requirements of an allosteric barbiturate binding site on acetylcholine receptor-rich membranes isolated from Torpedo electroplaques have been characterized by the ability of fourteen barbiturates to displace [14C]-amobarbitone binding. 2. The barbiturates could be grouped into two classes with ten barbiturates producing a strong inhibition of [14C]-amobarbitone binding (class one) and with four exerting minimal effects (class two). 3. Eight of the ten class one barbiturates displaced essentially all of the [14C]-amobarbitone from its binding site, while, at their respective aqueous solubility limits, two of these barbiturates (thiopentone and dimethylbutylbarbitone (DMBB) inhibited [14C]-amobarbitone binding by nearly 80%. The apparent inhibition constants (KI) for the class one barbiturates ranged from 13 microM for amobarbitone to 2.8 mM for barbitone with the other eight agents lying in the range 100-600 microM, and having the rank order pentobarbitone approximately secobarbitone greater than thiopentone greater than DMBB greater than butabarbitone approximately phenobarbitone greater than aprobarbitone greater than allylbarbitone. 4. By contrast, the class two barbiturates had minimal effects even at close to saturating concentrations. [14C]-amobarbitone binding was reduced slightly (less than 30%) by hexobarbitone, mephobarbitone and methohexitone and was enhanced slightly (less than 20%) by metharbitone. 5. All of the class two, but none of the class one barbiturates, were N-methylated.
Full text
PDF
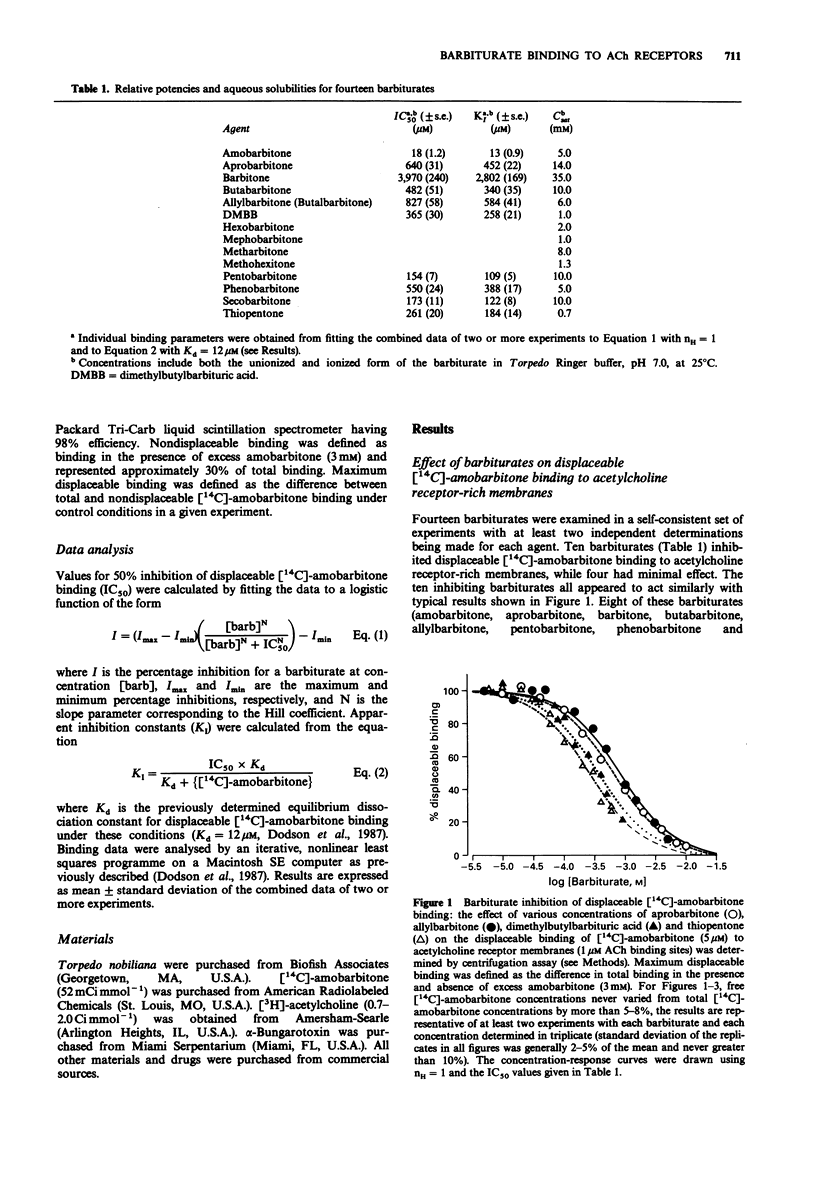
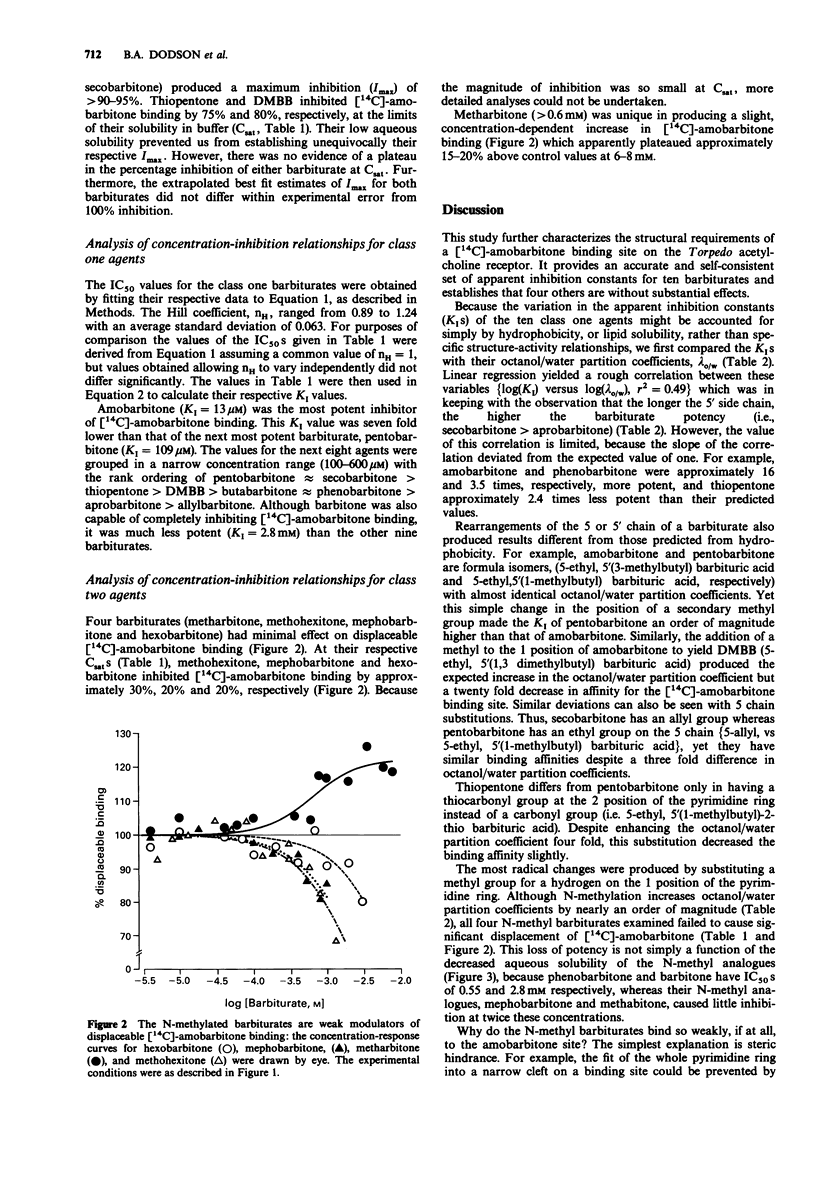
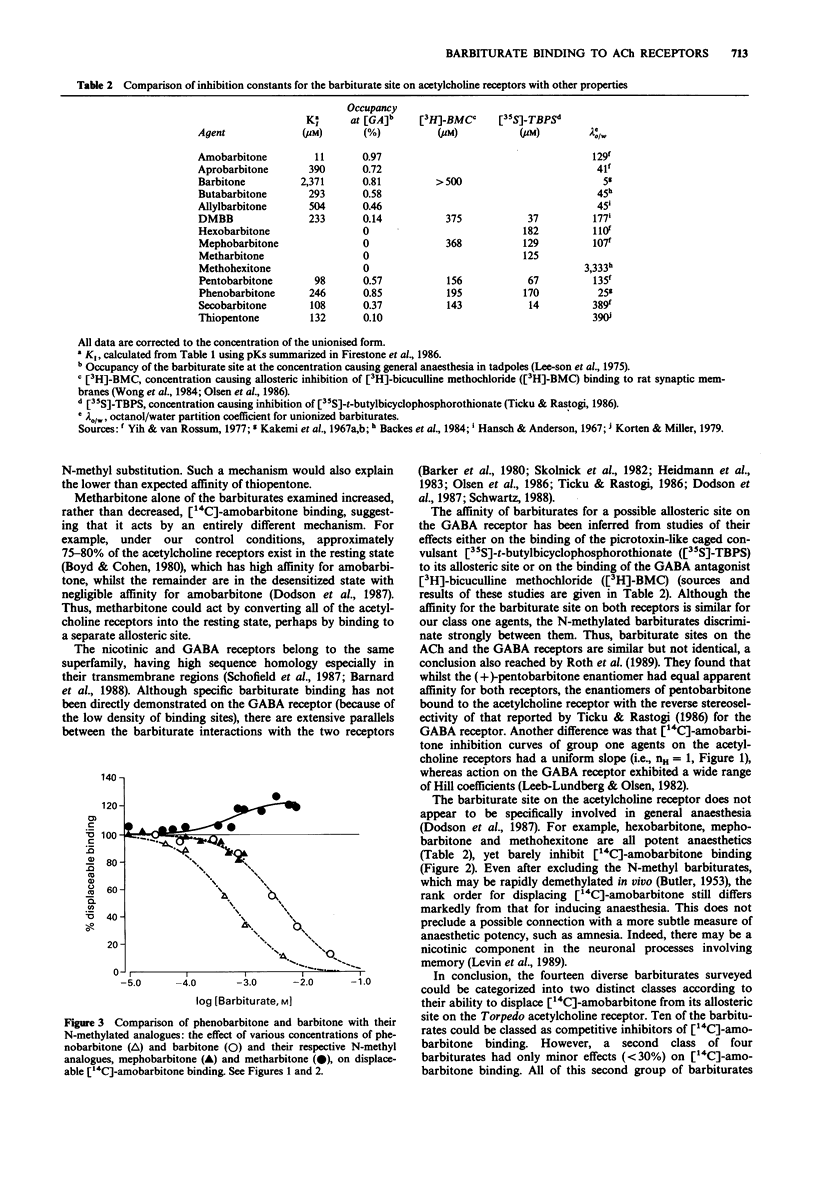

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER T. C. Quantitative studies of the demethylation of N-methyl barbital (metharbital, gemonil). J Pharmacol Exp Ther. 1953 Aug;108(4):474–480. [PubMed] [Google Scholar]
- Barnard E. A., Darlison M. G., Fujita N., Glencorse T. A., Levitan E. S., Reale V., Schofield P. R., Seeburg P. H., Squire M. D., Stephenson F. A. Molecular biology of the GABAA receptor. Adv Exp Med Biol. 1988;236:31–45. doi: 10.1007/978-1-4757-5971-6_3. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., Cohen J. B. Kinetics of binding of [3H]acetylcholine to Torpedo postsynaptic membranes: association and dissociation rate constants by rapid mixing and ultrafiltration. Biochemistry. 1980 Nov 11;19(23):5353–5358. doi: 10.1021/bi00564a032. [DOI] [PubMed] [Google Scholar]
- Dodson B. A., Braswell L. M., Miller K. W. Barbiturates bind to an allosteric regulatory site on nicotinic acetylcholine receptor-rich membranes. Mol Pharmacol. 1987 Jul;32(1):119–126. [PubMed] [Google Scholar]
- Gage P. W., McKinnon D. Effects of pentobarbitone on acetylcholine-activated channels in mammalian muscle. Br J Pharmacol. 1985 May;85(1):229–235. doi: 10.1111/j.1476-5381.1985.tb08851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansch C., Anderson S. M. The structure-activity relationship in barbiturates and its similarity to that in other narcotics. J Med Chem. 1967 Sep;10(5):745–753. doi: 10.1021/jm00317a001. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Oswald R. E., Changeux J. P. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Ho I. K., Harris R. A. Mechanism of action of barbiturates. Annu Rev Pharmacol Toxicol. 1981;21:83–111. doi: 10.1146/annurev.pa.21.040181.000503. [DOI] [PubMed] [Google Scholar]
- Kakemi K., Arita T., Hori R., Konishi R. Absorption and excretion of drugs. XXX. Absorption of barbituric acid derivatives from rat stomach. Chem Pharm Bull (Tokyo) 1967 Oct;15(10):1534–1539. doi: 10.1248/cpb.15.1534. [DOI] [PubMed] [Google Scholar]
- Kakemi K., Arita T., Hori R., Konishi R. Absorption and excretion of drugs. XXXI. On the relationship between partition coefficients and chemical structures of barbituric acid derivatives. Chem Pharm Bull (Tokyo) 1967 Nov;15(11):1705–1712. doi: 10.1248/cpb.15.1705. [DOI] [PubMed] [Google Scholar]
- Korten K., Miller K. W. Erythrocyte ghost--buffer partition coefficients of phenobarbital, pentobarbital, and thiopental support the pH-partition hypothesis. Can J Physiol Pharmacol. 1979 Mar;57(3):325–328. doi: 10.1139/y79-050. [DOI] [PubMed] [Google Scholar]
- Lee-Son S., Waud B. E., Waud D. R. A comparison of the potencies of a series of barbiturates at the neuromuscular junction and on the central nervous system. J Pharmacol Exp Ther. 1975 Nov;195(2):251–256. [PubMed] [Google Scholar]
- Leeb-Lundberg F., Olsen R. W. Interactions of barbiturates of various pharmacological categories with benzodiazepine receptors. Mol Pharmacol. 1982 Mar;21(2):320–328. [PubMed] [Google Scholar]
- Levin E. D., McGurk S. R., South D., Butcher L. L. Effects of combined muscarinic and nicotinic blockade on choice accuracy in the radial-arm maze. Behav Neural Biol. 1989 Mar;51(2):270–277. doi: 10.1016/s0163-1047(89)90917-5. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Richter J. A., Holtman J. R., Jr Barbiturates: their in vivo effects and potential biochemical mechanisms. Prog Neurobiol. 1982;18(4):275–319. doi: 10.1016/0301-0082(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Roth S. H., Forman S. A., Braswell L. M., Miller K. W. Actions of pentobarbital enantiomers on nicotinic cholinergic receptors. Mol Pharmacol. 1989 Dec;36(6):874–880. [PubMed] [Google Scholar]
- Schwartz R. D. The GABAA receptor-gated ion channel: biochemical and pharmacological studies of structure and function. Biochem Pharmacol. 1988 Sep 15;37(18):3369–3375. doi: 10.1016/0006-2952(88)90684-3. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A., Turner J. P. Potentiators of responses to activation of gamma-aminobutyric acid (GABAA) receptors. Neuropharmacology. 1987 Jul;26(7B):923–930. doi: 10.1016/0028-3908(87)90071-2. [DOI] [PubMed] [Google Scholar]
- Skolnick P., Rice K. C., Barker J. L., Paul S. M. Interaction of barbiturates with benzodiazepine receptors in the central nervous system. Brain Res. 1982 Feb 4;233(1):143–156. doi: 10.1016/0006-8993(82)90936-2. [DOI] [PubMed] [Google Scholar]
- Willow M., Johnston G. A. Pharmacology of barbiturates: electrophysiological and neurochemical studies. Int Rev Neurobiol. 1983;24:15–49. doi: 10.1016/s0074-7742(08)60219-6. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Snowman A. M., Leeb-Lundberg L. M., Olsen R. W. Barbiturates allosterically inhibit GABA antagonist and benzodiazepine inverse agonist binding. Eur J Pharmacol. 1984 Jul 13;102(2):205–212. doi: 10.1016/0014-2999(84)90252-8. [DOI] [PubMed] [Google Scholar]
- Yih T. D., van Rossum J. M. K5 values of some homologous series of barbiturates and the relationship with the lipophilicity and metabolic clearance. Biochem Pharmacol. 1977 Nov 15;26(22):2117–2120. doi: 10.1016/0006-2952(77)90261-1. [DOI] [PubMed] [Google Scholar]


