Abstract
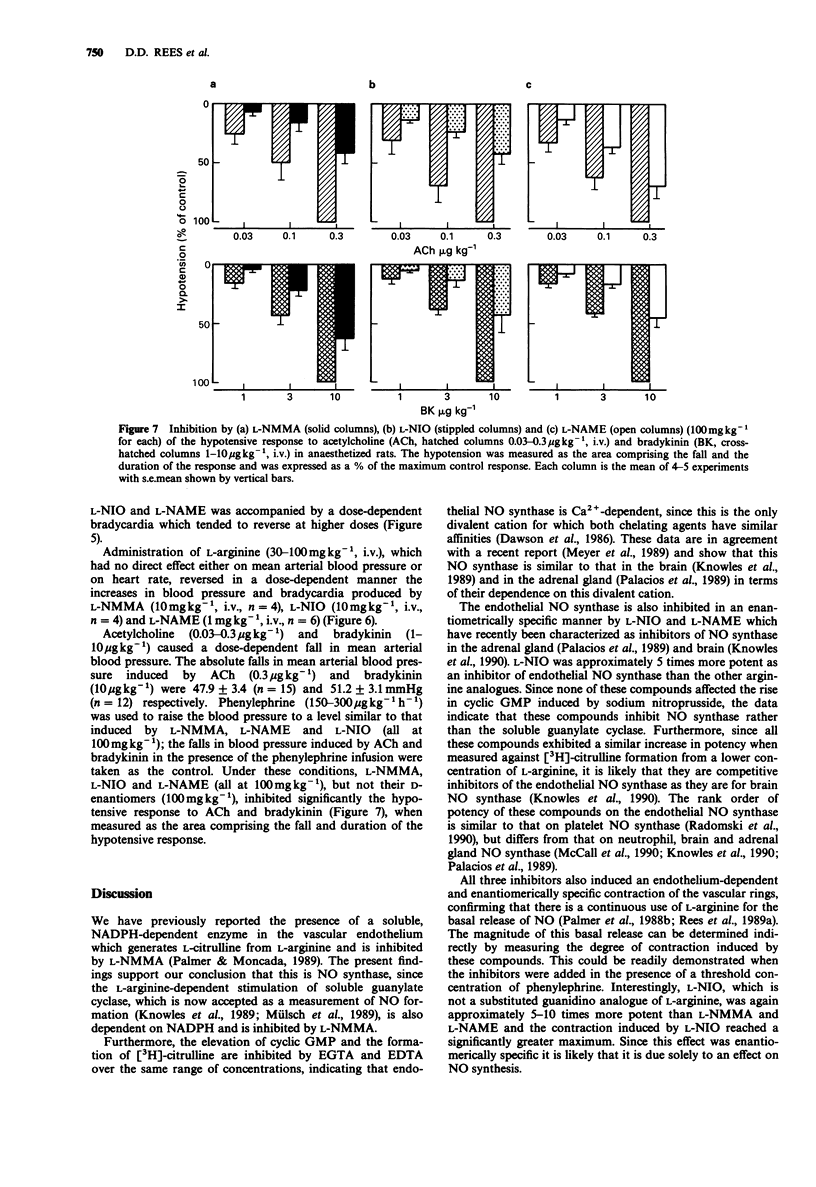
1. Three analogues of L-arginine were characterized as inhibitors of endothelial nitric oxide (NO) synthase by measuring their effect on the endothelial NO synthase from porcine aortae, on the vascular tone of rings of rat aorta and on the blood pressure of the anaesthetized rat. 2. NG-monomethyl-L-arginine (L-NMMA), N-iminoethyl-L-ornithine (L-NIO) and NG-nitro-L-arginine methyl ester (L-NAME; all at 0.1-100 microM) caused concentration-dependent inhibition of the Ca2(+)-dependent endothelial NO synthase from porcine aortae. 3. L-NMMA, L-NIO and L-NAME caused an endothelium-dependent contraction and an inhibition of the endothelium-dependent relaxation induced by acetylcholine (ACh) in aortic rings. 4. L-NMMA, L-NIO and L-NAME (0.03-300 mg kg-1, i.v.) induced a dose-dependent increase in mean systemic arterial blood pressure accompanied by bradycardia. 5. L-NMMA, L-NIO and L-NAME (100 mg kg-1, i.v.) inhibited significantly the hypotensive responses to ACh and bradykinin. 6. The increase in blood pressure and bradycardia produced by these compounds were reversed by L-arginine (30-100 mg kg-1, i.v.) in a dose-dependent manner. 7. All of these effects were enantiomer specific. 8. These results indicate that L-NMMA, L-NIO and L-NAME are inhibitors of NO synthase in the vascular endothelium and confirm the important role of NO synthesis in the maintenance of vascular tone and blood pressure.
Full text
PDF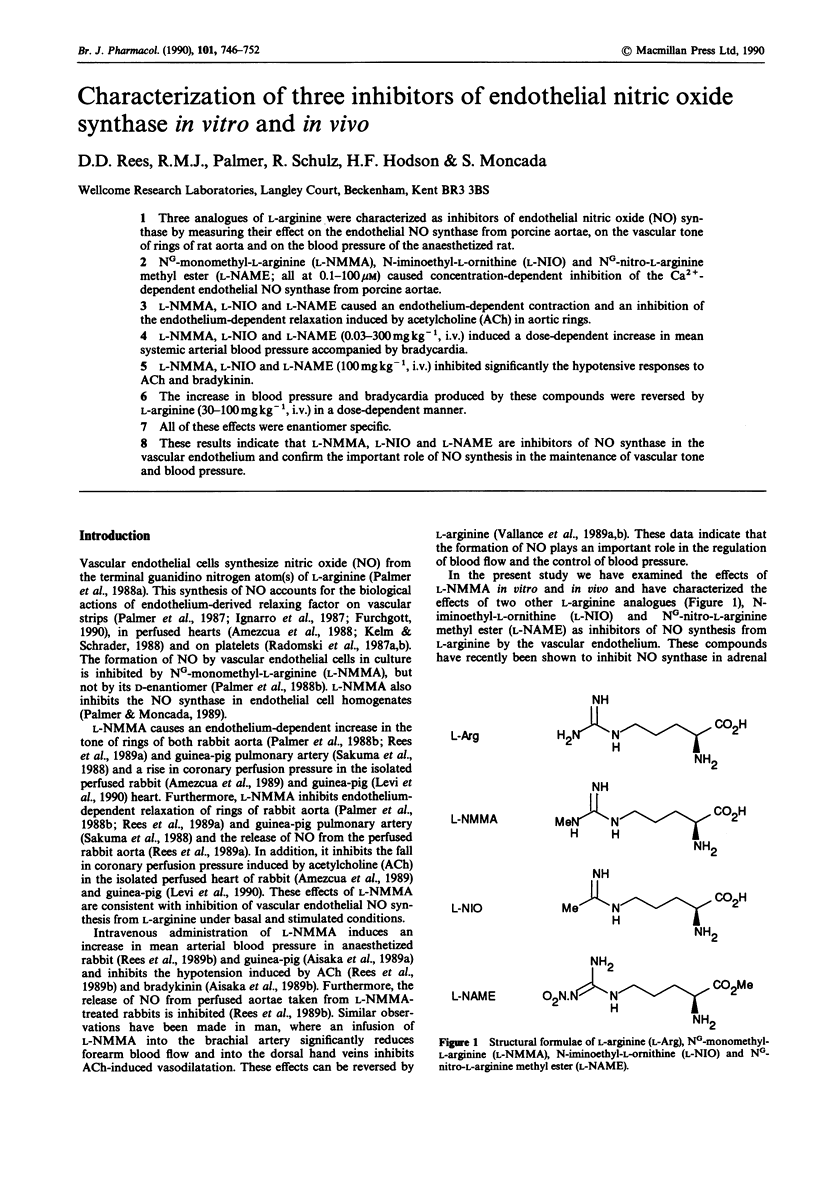





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- Aisaka K., Gross S. S., Griffith O. W., Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun. 1989 Apr 28;160(2):881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- Amezcua J. L., Dusting G. J., Palmer R. M., Moncada S. Acetylcholine induces vasodilatation in the rabbit isolated heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988 Nov;95(3):830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua J. L., Palmer R. M., de Souza B. M., Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989 Aug;97(4):1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascariasis. Lancet. 1989 May 6;1(8645):997–998. [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Palmer R. M., Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989 Oct 17;172(4-5):413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Kinetic characteristics of nitric oxide synthase from rat brain. Biochem J. 1990 Jul 1;269(1):207–210. doi: 10.1042/bj2690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Hattori K. Nitroarginine inhibits endothelium-derived relaxation. Jpn J Pharmacol. 1990 Jan;52(1):167–169. doi: 10.1254/jjp.52.167. [DOI] [PubMed] [Google Scholar]
- Koulu M., Lappalainen J., Pesonen U., Hietala J., Syvälahti E. Chronic treatment with SCH 23390, a selective dopamine D-1 receptor antagonist, decreases dopamine metabolism in rat caudate nucleus. Eur J Pharmacol. 1988 Oct 18;155(3):313–316. doi: 10.1016/0014-2999(88)90521-3. [DOI] [PubMed] [Google Scholar]
- Mayer B., Schmidt K., Humbert P., Böhme E. Biosynthesis of endothelium-derived relaxing factor: a cytosolic enzyme in porcine aortic endothelial cells Ca2+-dependently converts L-arginine into an activator of soluble guanylyl cyclase. Biochem Biophys Res Commun. 1989 Oct 31;164(2):678–685. doi: 10.1016/0006-291x(89)91513-1. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Palacios M., Knowles R. G., Palmer R. M., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989 Dec 15;165(2):802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Patthy A., Bajusz S., Patthy L. Preparation and characterization of Ng-mono-, di- and trimethylated arginines. Acta Biochim Biophys Acad Sci Hung. 1977;12(3):191–196. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell J. P., Ax H. A., Pruess D. L., Williams T., Demny T. C. Antimetabolites produced by microorganisms. VI. L-N 5 -(1-iminoethyl) ornithine. J Antibiot (Tokyo) 1972 Mar;25(3):179–184. doi: 10.7164/antibiotics.25.179. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Nitric oxide synthesised from L-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc Res. 1989 Dec;23(12):1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]


