Abstract
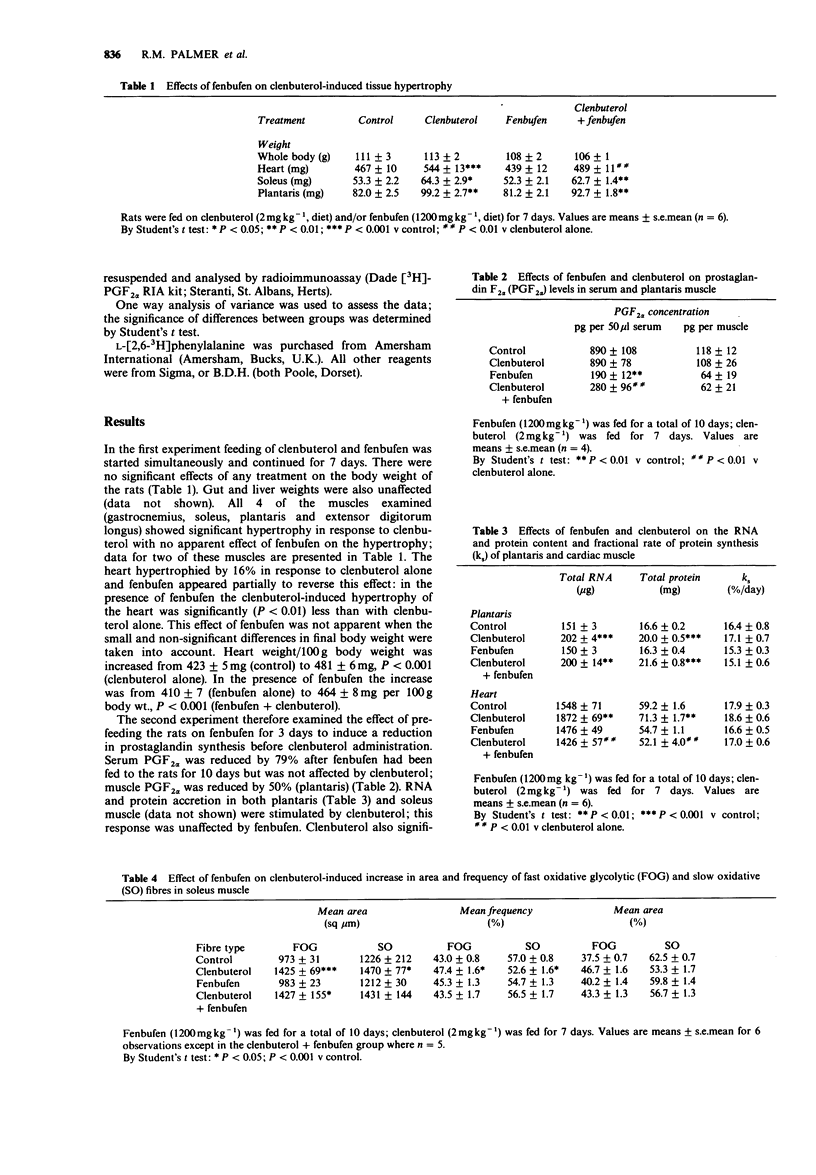
1. When rats were fed with clenbuterol for 7 days skeletal muscle mass increased by 21% in the tonic soleus and phasic plantaris muscles and a 16% hypertrophy of the heart was also induced. Fenbufen, fed to rats for the same period, blocked the hypertrophy of the heart but not that of the skeletal muscles. 2. When feeding of fenbufen commenced 3 days before the administration of clenbuterol, plasma prosta-glandin F2 alpha (PGF2 alpha) was reduced by 79%; there was again no effect of fenbufen on clenbuterol-induced increases in the RNA or protein content of plantaris, nor in the increased area of fast or slow twitch fibres in the soleus. In the heart the clenbuterol-induced increases in the RNA (+21%) and protein content (+20%) were totally inhibited. 3. The effects of clenbuterol on heart muscle appear to be mediated by a cyclo-oxygenase metabolite of arachidonic acid whilst the effects on skeletal muscle are not.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson M. M., Millward D. J. Effect of the cyclo-oxygenase inhibitor fenbufen on muscle and liver protein metabolism, muscle glutamine and plasma insulin in endotoxaemic rats. Clin Sci (Lond) 1989 Jul;77(1):13–20. doi: 10.1042/cs0770013. [DOI] [PubMed] [Google Scholar]
- MacLennan P. A., Edwards R. H. Effects of clenbuterol and propranolol on muscle mass. Evidence that clenbuterol stimulates muscle beta-adrenoceptors to induce hypertrophy. Biochem J. 1989 Dec 1;264(2):573–579. doi: 10.1042/bj2640573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltin C. A., Delday M. I., Reeds P. J. The effect of a growth promoting drug, clenbuterol, on fibre frequency and area in hind limb muscles from young male rats. Biosci Rep. 1986 Mar;6(3):293–299. doi: 10.1007/BF01115158. [DOI] [PubMed] [Google Scholar]
- Maltin C. A., Hay S. M., Delday M. I., Reeds P. J., Palmer R. M. Evidence that the hypertrophic action of clenbuterol on denervated rat muscle is not propranolol-sensitive. Br J Pharmacol. 1989 Apr;96(4):817–822. doi: 10.1111/j.1476-5381.1989.tb11889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltin C. A., Hay S. M., Delday M. I., Smith F. G., Lobley G. E., Reeds P. J. Clenbuterol, a beta agonist, induces growth in innervated and denervated rat soleus muscle via apparently different mechanisms. Biosci Rep. 1987 Jun;7(6):525–532. doi: 10.1007/BF01116510. [DOI] [PubMed] [Google Scholar]
- McElligott M. A., Mulder J. E., Chaung L. Y., Barreto A., Jr Clenbuterol-induced muscle growth: investigation of possible mediation by insulin. Am J Physiol. 1987 Oct;253(4 Pt 1):E370–E375. doi: 10.1152/ajpendo.1987.253.4.E370. [DOI] [PubMed] [Google Scholar]
- McMillan D. N., Reeds P. J., Lobley G. E., Palmer R. M. Changes in protein turnover in hypertrophying plantaris muscles of rats: effect of fenbufen--an inhibitor of prostaglandin synthesis. Prostaglandins. 1987 Dec;34(6):841–852. doi: 10.1016/0090-6980(87)90065-7. [DOI] [PubMed] [Google Scholar]
- Pullar J. D., Webster A. J. The energy cost of fat and protein deposition in the rat. Br J Nutr. 1977 May;37(3):355–363. doi: 10.1079/bjn19770039. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Hay S. M., Dorward P. M., Palmer R. M. The effect of beta-agonists and antagonists on muscle growth and body composition of young rats (Rattus sp.). Comp Biochem Physiol C. 1988;89(2):337–341. doi: 10.1016/0742-8413(88)90234-4. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Hay S. M., Dorwood P. M., Palmer R. M. Stimulation of muscle growth by clenbuterol: lack of effect on muscle protein biosynthesis. Br J Nutr. 1986 Jul;56(1):249–258. doi: 10.1079/bjn19860104. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Hay S. M., Glennie R. T., Mackie W. S., Garlick P. J. The effect of indomethacin on the stimulation of protein synthesis by insulin in young post-absorptive rats. Biochem J. 1985 Apr 1;227(1):255–261. doi: 10.1042/bj2270255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds P. J., Palmer R. M. The possible involvement of prostaglandin F2 alpha in the stimulation of muscle protein synthesis by insulin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1084–1090. doi: 10.1016/s0006-291x(83)80253-8. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Ruff R. L., Secrist D. Inhibitors of prostaglandin synthesis or cathepsin B prevent muscle wasting due to sepsis in the rat. J Clin Invest. 1984 May;73(5):1483–1486. doi: 10.1172/JCI111352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Sugden P. H. Effects of pressure overload and insulin on protein turnover in the perfused rat heart. Prostaglandins are not involved although their synthesis is stimulated by insulin. Biochem J. 1987 Apr 15;243(2):473–479. doi: 10.1042/bj2430473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. H., Palmer R. M., Reeds P. J. Protein synthesis in isolated rabbit forelimb muscles. The possible role of metabolites of arachidonic acid in the response to intermittent stretching. Biochem J. 1983 Jul 15;214(1):153–161. doi: 10.1042/bj2140153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Baracos V. E. Prostaglandin-dependent muscle wasting during infection in the broiler chick (Gallus domesticus) and the laboratory rat (Rattus norvegicus). Biochem J. 1989 Oct 15;263(2):485–490. doi: 10.1042/bj2630485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster B., Vigna S. R., Paquette T., Koerker D. J. Beta-adrenergic modulation of insulin binding in skeletal muscle. Am J Physiol. 1986 Feb;250(2 Pt 1):E198–E204. doi: 10.1152/ajpendo.1986.250.2.E198. [DOI] [PubMed] [Google Scholar]
- Zeman R. J., Ludemann R., Easton T. G., Etlinger J. D. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a beta 2-receptor agonist. Am J Physiol. 1988 Jun;254(6 Pt 1):E726–E732. doi: 10.1152/ajpendo.1988.254.6.E726. [DOI] [PubMed] [Google Scholar]



