Abstract
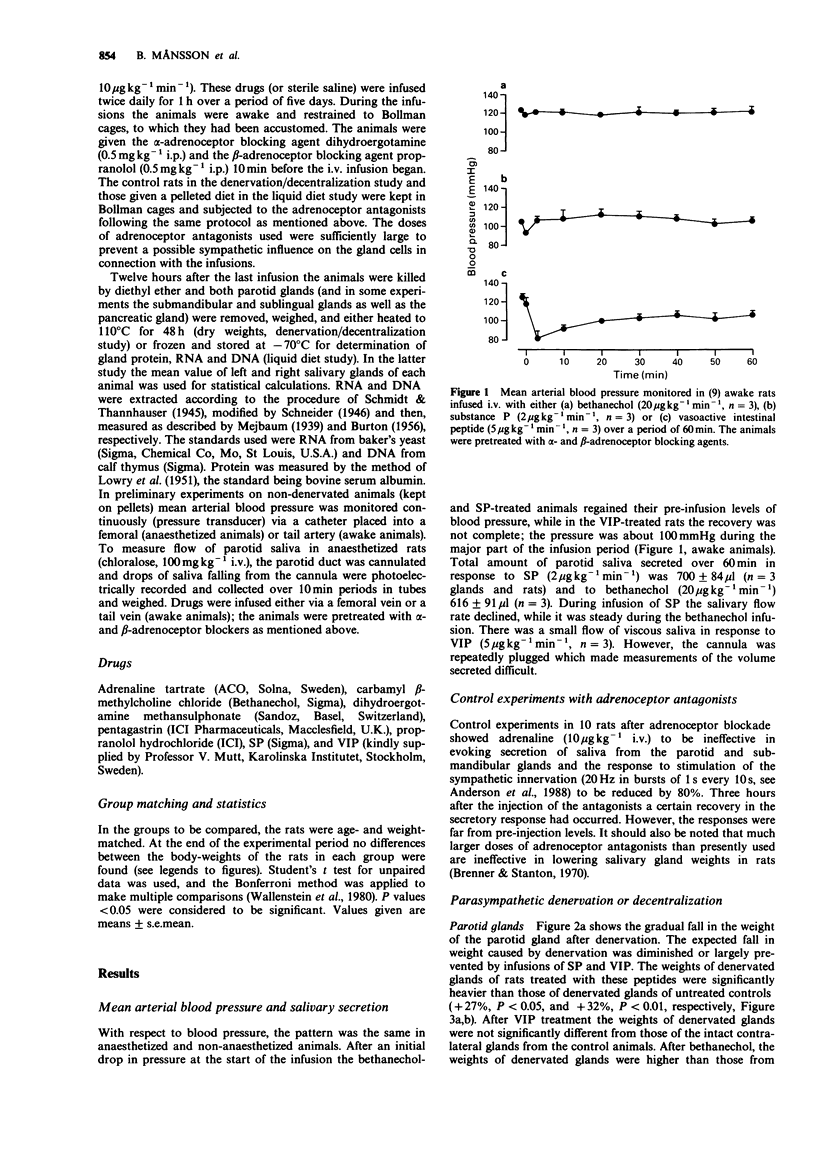
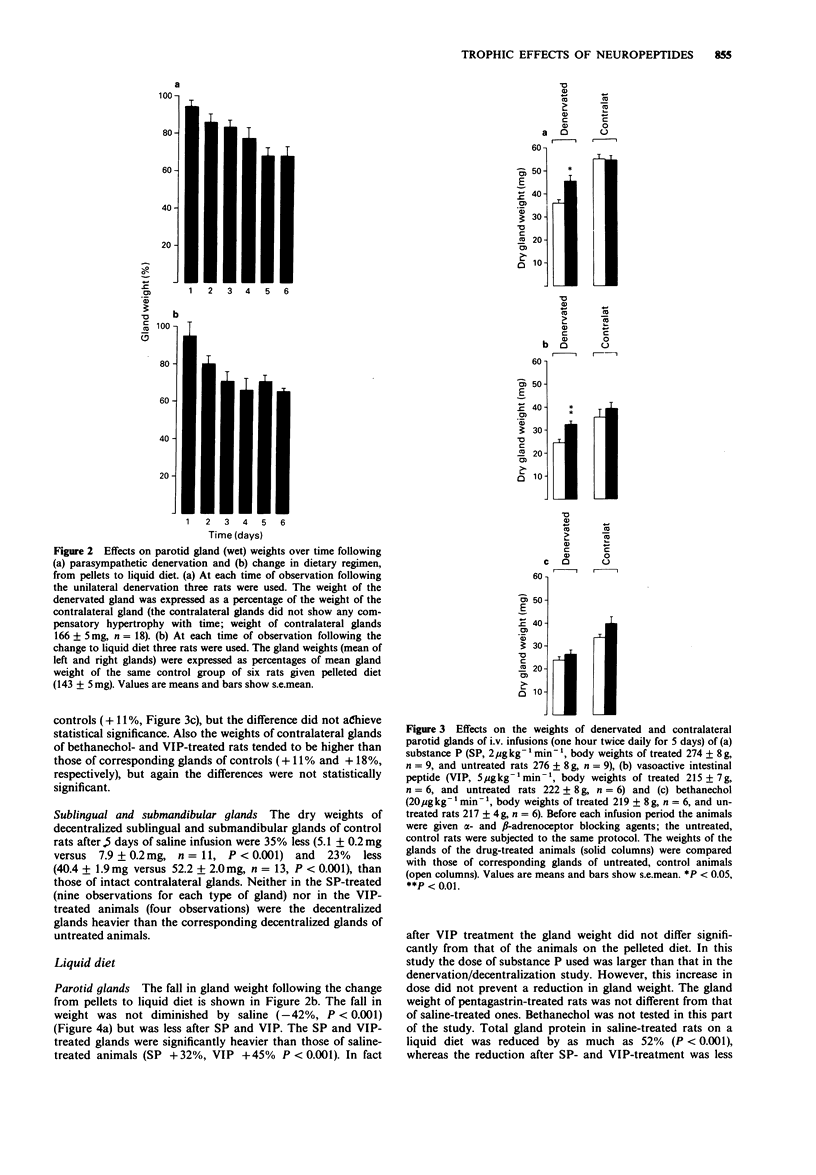
1. The long-term influence of substance P (SP) and vasoactive intestinal peptide (VIP) on rat salivary gland weight was investigated after parasympathetic denervation or on feeding soft food. 2. The parotid gland lost about one-third of its weight within 4-5 days following parasympathetic post-ganglionic denervation or change in dietary regimen, from pellets to liquid diet, thought to reduce nerve reflex activity. 3. Daily i.v. infusions with SP or VIP diminished or largely prevented the fall in parotid gland weight, whereas infusions with pentagastrin, bethanechol and saline had no effect. The infusions were preceded by administration of alpha- and beta-adrenoceptor antagonists; these antagonists were also given to the control animals. 4. The effect of SP and VIP on the parotid gland weight appeared to be related to cell size rather than to cell number, as judged by measurements of RNA and DNA. 5. Observations on the two other major salivary glands underlined the fact that different gland types in the same animal behave differently. Parasympathetic preganglionic denervation (decentralization) lowered the weights of the sublingual and submandibular glands, whereas liquid diet only reduced the weight of the sublingual gland. SP and VIP did not affect the weights of the submandibular glands, but VIP prevented the slight fall in sublingual gland weight induced by liquid diet. 6. The present results suggest a trophic role in rats for SP and VIP on parotid glands and for VIP on sublingual glands. Such an influence may be exerted naturally as a result of their release from nerves containing these peptides around acini.
Full text
PDF
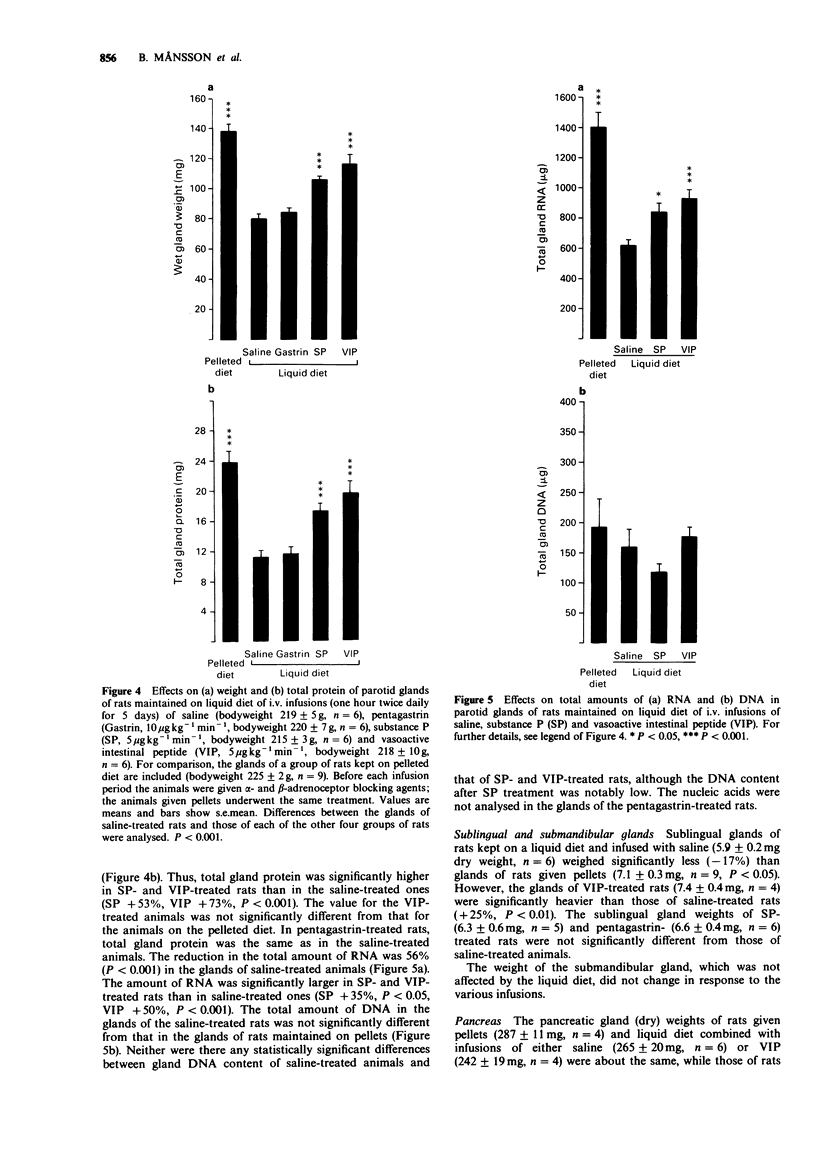


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm P., Ekström J. Cholinergic nerves of unknown origin in the parotid glands of rats. Arch Oral Biol. 1976;21(7):417–421. doi: 10.1016/0003-9969(76)90005-4. [DOI] [PubMed] [Google Scholar]
- Anderson L. C., Garrett J. R., Proctor G. B. Advantages of burst stimulation for inducing sympathetic salivary secretion in rats. Q J Exp Physiol. 1988 Nov;73(6):1025–1028. doi: 10.1113/expphysiol.1988.sp003215. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaccini G., De Caro G., Cheli R. Enlargement of salivary glands in rats after chronic administration of physalaemin or isoprenaline. J Pharm Pharmacol. 1966 May;18(5):312–316. doi: 10.1111/j.2042-7158.1966.tb07877.x. [DOI] [PubMed] [Google Scholar]
- Brenner G. M., Stanton H. C. Adrenergic mechanism responsible for submandibular salivary glandular hypertrophy in the rat. J Pharmacol Exp Ther. 1970 May;173(1):166–175. [PubMed] [Google Scholar]
- Butcher F. R., Putney J. W., Jr Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. [PubMed] [Google Scholar]
- Cantalamessa F., de Caro G., Perfumi M. Effects of chronic administration of eldoisin or physalaemin on the rat salivary glands. Pharmacol Res Commun. 1975 Jun;7(3):259–271. doi: 10.1016/0031-6989(75)90025-9. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Hultgårdh-Nilsson A., Haegerstrand A., Nilsson J. Neuropeptides as growth factors. Possible roles in human diseases. Regul Pept. 1989 Apr;25(1):1–9. doi: 10.1016/0167-0115(89)90243-7. [DOI] [PubMed] [Google Scholar]
- Dehaye J. P., Christophe J., Ernst F., Poloczek P., Van Bogaert P. Binding in vitro of vasoactive intestinal peptide on isolated acini of rat parotid glands. Arch Oral Biol. 1985;30(11-12):827–832. doi: 10.1016/0003-9969(85)90139-6. [DOI] [PubMed] [Google Scholar]
- Ekström J., Brodin E., Ekman R., Håkanson R., Sundler F. Vasoactive intestinal peptide and substance P in salivary glands of the rat following denervation or duct ligation. Regul Pept. 1984 Dec;10(1):1–10. doi: 10.1016/0167-0115(84)90047-8. [DOI] [PubMed] [Google Scholar]
- Ekström J. Choline acetyltransferase activity in parotid glands of rats after prolonged treatment with pilocarpine. Acta Physiol Scand. 1977 Jan;99(1):110–112. doi: 10.1111/j.1748-1716.1977.tb10359.x. [DOI] [PubMed] [Google Scholar]
- Ekström J. Choline acetyltransferase activity in rat salivary glands after cellulose rich diet or treatment with an atropine-like drug. Q J Exp Physiol Cogn Med Sci. 1974 Jul;59(3):191–199. doi: 10.1113/expphysiol.1974.sp002261. [DOI] [PubMed] [Google Scholar]
- Ekström J., Ekman R., Håkanson R., Luts A., Sundler F., Tobin G. Effects of capsaicin pretreatment on neuropeptides and salivary secretion of rat parotid glands. Br J Pharmacol. 1989 Aug;97(4):1031–1038. doi: 10.1111/j.1476-5381.1989.tb12559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström J., Månsson B., Nilsson B. O., Rosengren E., Tobin G. Receptors involved in the nervous system regulation of polyamine metabolism in rat salivary glands. Acta Physiol Scand. 1989 Mar;135(3):255–261. doi: 10.1111/j.1748-1716.1989.tb08575.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Månsson B., Tobin G., Garrett J. R., Thulin A. Atropine-resistant secretion of parotid saliva on stimulation of the auriculo-temporal nerve. Acta Physiol Scand. 1983 Dec;119(4):445–449. doi: 10.1111/j.1748-1716.1983.tb07360.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Månsson B., Tobin G. Vasoactive intestinal peptide evoked secretion of fluid and protein from rat salivary glands and the development of supersensitivity. Acta Physiol Scand. 1983;119(2):169–175. doi: 10.1111/j.1748-1716.1983.tb07322.x. [DOI] [PubMed] [Google Scholar]
- Ekström J. Neuropeptides and secretion. J Dent Res. 1987 Feb;66(2):524–530. doi: 10.1177/00220345870660022301. [DOI] [PubMed] [Google Scholar]
- Ekström J., Nilsson B. O., Rosengren E. Substance P and vasoactive intestinal peptide influence polyamine metabolism in salivary glands of the rat. Acta Physiol Scand. 1989 Jul;136(3):427–433. doi: 10.1111/j.1748-1716.1989.tb08684.x. [DOI] [PubMed] [Google Scholar]
- Ekström J. Sensitization of the rat parotid gland to secretagogues following either parasympathetic denervation or sympathetic denervation or decentralization. Acta Physiol Scand. 1980 Mar;108(3):253–261. doi: 10.1111/j.1748-1716.1980.tb06531.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Templeton D. Difference in sensitivity of parotid glands brought about by disuse and overuse. Acta Physiol Scand. 1977 Nov;101(3):329–335. doi: 10.1111/j.1748-1716.1977.tb06014.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Wahlestedt C. Supersensitivity to substance P and physalaemin in rat salivary glands after denervation or decentralization. Acta Physiol Scand. 1982 Aug;115(4):437–446. doi: 10.1111/j.1748-1716.1982.tb07102.x. [DOI] [PubMed] [Google Scholar]
- HOUSSAY A. B., PERONACE A. A., PEREC C. J., RUBINSTEIN O. Role of the chorda tympani in submaxillary hypertrophy by incisor amputation in the rat. Acta Physiol Lat Am. 1962;12:153–166. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malhotra R. K., Wakade A. R. Vasoactive intestinal polypeptide stimulates the secretion of catecholamines from the rat adrenal gland. J Physiol. 1987 Jul;388:285–294. doi: 10.1113/jphysiol.1987.sp016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayston P. D., Barrowman J. A. The influence of chronic administration of pentagastrin on the rat pancreas. Q J Exp Physiol Cogn Med Sci. 1971 Apr;56(2):113–122. doi: 10.1113/expphysiol.1971.sp002105. [DOI] [PubMed] [Google Scholar]
- Månsson B., Ekman R., Håkanson R., Ekström J. Neuropeptides and disuse of the rat parotid gland. Exp Physiol. 1990 Jul;75(4):597–599. doi: 10.1113/expphysiol.1990.sp003435. [DOI] [PubMed] [Google Scholar]
- Ohlin P., Perec C. The effect of treatment with parasympatholytics on the weight of the submaxillary gland of rats. Experientia. 1966 Oct 15;22(10):668–669. doi: 10.1007/BF01902434. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 1986;48:75–88. doi: 10.1146/annurev.ph.48.030186.000451. [DOI] [PubMed] [Google Scholar]
- SCHNEYER C. A. Salivary gland changes after isoproterenol-induced enlargement. Am J Physiol. 1962 Aug;203:232–236. doi: 10.1152/ajplegacy.1962.203.2.232. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A. Beta-adrenergic effects by autonomic agents on mitosis and hypertrophy in rat parotid. Proc Soc Exp Biol Med. 1969 May;131(1):71–75. doi: 10.3181/00379727-131-33806. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A., Hall H. D. Function of rat parotid gland after sympathectomy and total postganglionectomy. Am J Physiol. 1966 Oct;211(4):943–949. doi: 10.1152/ajplegacy.1966.211.4.943. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A. Mitosis induced in adult rat parotid following normal activity of the gland. Proc Soc Exp Biol Med. 1970 May;134(1):98–102. doi: 10.3181/00379727-134-34737. [DOI] [PubMed] [Google Scholar]
- Sharkey K. A., Templeton D. Substance P in the rat parotid gland: evidence for a dual origin from the otic and trigeminal ganglia. Brain Res. 1984 Jun 25;304(2):392–396. doi: 10.1016/0006-8993(84)90346-9. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Uddman R., Fahrenkrug J., Malm L., Alumets J., Håkanson R., Sundler F. Neuronal VIP in salivary glands: distribution and release. Acta Physiol Scand. 1980 Sep;110(1):31–38. doi: 10.1111/j.1748-1716.1980.tb06626.x. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wells H., Peronace A. A. Functional hypertrophy and atrophy of the salivary glands of rats. Am J Physiol. 1967 Feb;212(2):247–251. doi: 10.1152/ajplegacy.1967.212.2.247. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Bryant M. G., Van Noorden S., Bloom S. R., Pearse A. G. Vasoactive intestinal polypeptide (VIP)-like immunoreactivity in salivary glands. Life Sci. 1979 Jul 16;25(3):273–280. doi: 10.1016/0024-3205(79)90295-9. [DOI] [PubMed] [Google Scholar]



