Abstract
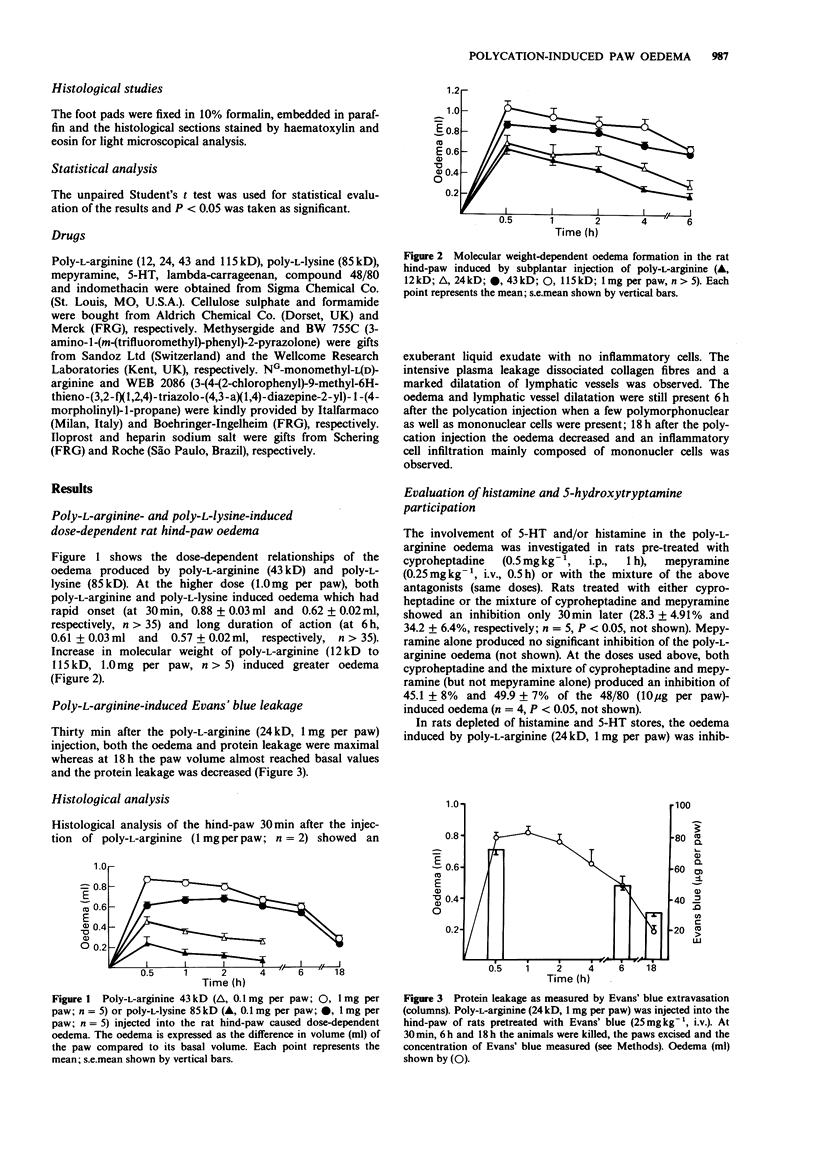
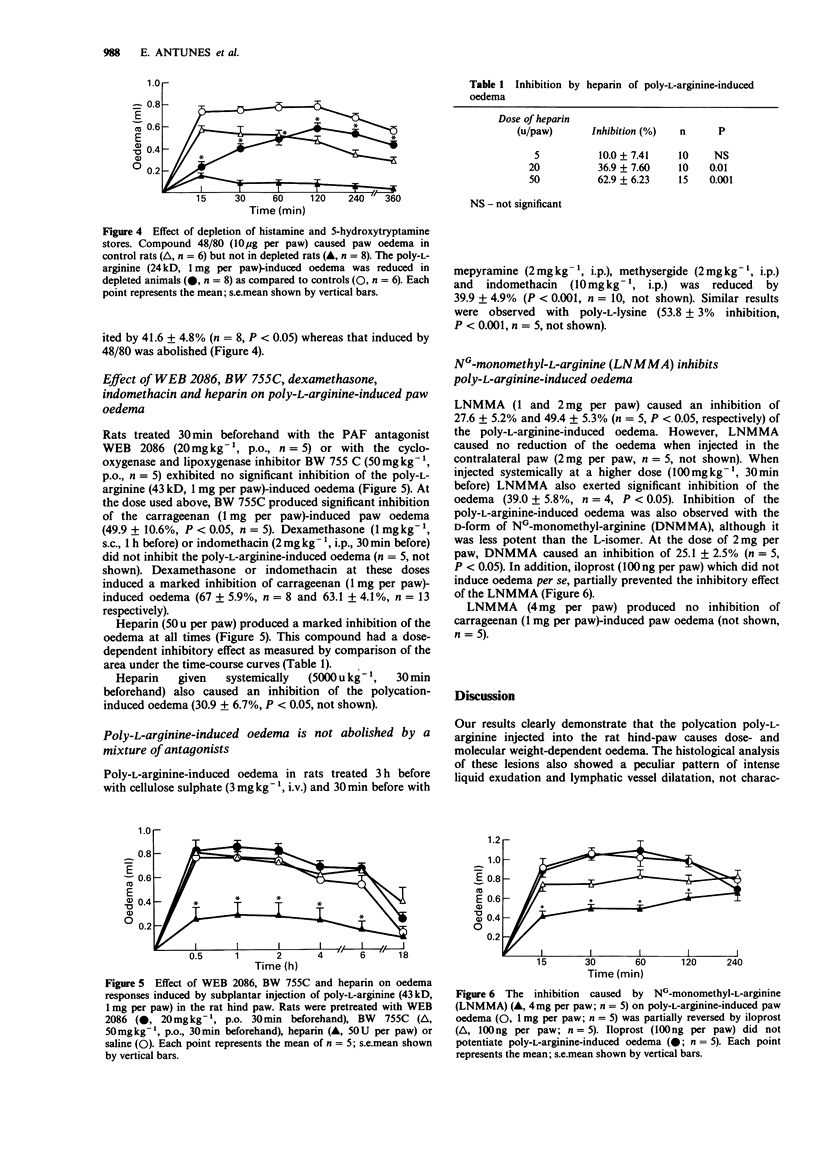
1. The inflammatory response induced by poly-L-arginine in the rat hind-paw was studied both by measuring paw oedema and histologically. 2. The paw volume was measured with a hydroplethysmometer at 0.5, 1, 2, 4, 6 and 18 h after the subplantar injection of the polycation. Protein extravasation was evaluated with Evans' blue and the histology studied by light microscopy. 3. Poly-L-arginine (12, 24, 43 and 115kD) caused dose- and molecular weight-dependent oedema which had a rapid onset and long duration. Evans' blue extravasation paralleled the oedema induced by poly-L-arginine. Microscopic examination of the paws at early stages of oedema formation showed exuberant liquid exudate with no inflammatory cells. After 18 h, a cellular infiltrate was present, consisting mainly of mononuclear cells. 4. Indomethacin, dexamethasone, BW755c or the PAF-antagonist WEB 2086 caused no significant inhibition of the poly-L-arginine-induced oedema. Cyproheptadine had inhibitory effects only on the early stages of the polycation-induced oedema. Similar results were observed with rats depleted of histamine and 5-hydroxytryptamine. 5. Heparin, a polyanion, injected in the rat paw caused a marked inhibition of the polycation-induced oedema. NG-monomethyl-L-arginine (LNMMA), an inhibitor of EDRF synthesis, injected locally also produced a marked inhibition, but this inhibition was reversed by iloprost. 6. These results suggest that the oedema induced by polycations was due to their cationic charge. The inhibitory effect of LNMMA is probably due to a decrease in vascular flow rather than a decrease in vascular permeability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes J. L., Radnik R. A., Gilchrist E. P., Venkatachalam M. A. Size and charge selective permeability defects induced in glomerular basement membrane by a polycation. Kidney Int. 1984 Jan;25(1):11–19. doi: 10.1038/ki.1984.2. [DOI] [PubMed] [Google Scholar]
- Barnes J. L., Venkatachalam M. A. Enhancement of glomerular immune complex deposition by a circulating polycation. J Exp Med. 1984 Jul 1;160(1):286–293. doi: 10.1084/jem.160.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bârzu T., Molho P., Tobelem G., Petitou M., Caen J. Binding and endocytosis of heparin by human endothelial cells in culture. Biochim Biophys Acta. 1985 May 30;845(2):196–203. doi: 10.1016/0167-4889(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Mazzucco G., Monga G., Roffinello C., Alberton M., Dellabona P., Malavasi F., Vercellone A. Platelet cationic proteins are present in glomeruli of lupus nephritis patients. Kidney Int. 1986 Oct;30(4):555–565. doi: 10.1038/ki.1986.221. [DOI] [PubMed] [Google Scholar]
- DE VRIES A., KATCHALSKI E., STEIN O. The effect of polyamino acids on the blood vessels of the rat. Arch Int Pharmacodyn Ther. 1956 Aug 1;107(2):243–253. [PubMed] [Google Scholar]
- Di Rosa M., Giroud J. P., Willoughby D. A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971 May;104(1):15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- Ennis M., Pearce F. L., Weston P. M. Some studies on the release of histamine from mast cells stimulated with polylysine. Br J Pharmacol. 1980 Oct;70(2):329–334. doi: 10.1111/j.1476-5381.1980.tb07940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Hiebert L. The uptake of heparin by liver sinusoidal cells in normal and atherosclerotic rabbits. 1981 Feb 15-Mar 1Thromb Res. 21(4-5):383–390. doi: 10.1016/0049-3848(81)90139-0. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Flower R. J., Vane J. R. A new approach to anti-inflammatory drugs. Biochem Pharmacol. 1979 Jun 15;28(12):1959–1961. doi: 10.1016/0006-2952(79)90651-8. [DOI] [PubMed] [Google Scholar]
- Ichimura S., Zama M. The interaction of 8-anilino-1-naphthalenesulfonate with polylysine and polyarginine. Biopolymers. 1977 Jul;16(7):1449–1464. doi: 10.1002/bip.1977.360160706. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Gold M. E., Buga G. M., Byrns R. E., Wood K. S., Chaudhuri G., Frank G. Basic polyamino acids rich in arginine, lysine, or ornithine cause both enhancement of and refractoriness to formation of endothelium-derived nitric oxide in pulmonary artery and vein. Circ Res. 1989 Feb;64(2):315–329. doi: 10.1161/01.res.64.2.315. [DOI] [PubMed] [Google Scholar]
- Kazimierczak W., Diamant B. Mechanisms of histamine release in anaphylactic and anaphylactoid reactions. Prog Allergy. 1978;24:295–365. [PubMed] [Google Scholar]
- Leme J. G., Wilhelm D. L. The effects of adrenalectomy and corticosterone on vascular permeability responses in the skin of the rat. Br J Exp Pathol. 1975 Oct;56(5):402–407. [PMC free article] [PubMed] [Google Scholar]
- Lesser M. L., Braun H. I., Helson L. Statistical methods for measuring and comparing treatment efficacies: applications to nude mice experimentation. Exp Cell Biol. 1980;48(2):126–137. doi: 10.1159/000162981. [DOI] [PubMed] [Google Scholar]
- Lykke A. W., Cummings R. Inflammation in healing. 1. Time-course and mediation of exudation in wound healing in the rat. Br J Exp Pathol. 1969 Jun;50(3):309–318. [PMC free article] [PubMed] [Google Scholar]
- Needham L., Hellewell P. G., Williams T. J., Gordon J. L. Endothelial functional responses and increased vascular permeability induced by polycations. Lab Invest. 1988 Oct;59(4):538–548. [PubMed] [Google Scholar]
- ROWLEY D. A., BENDITT E. P. 5-Hydroxytryptamine and histamine as mediators of the vascular injury produced by agents which damage mast cells in rats. J Exp Med. 1956 Apr 1;103(4):399–412. doi: 10.1084/jem.103.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPECTOR W. G., WILLOUGHBY D. A. The demonstration of the role of mediators in turpentine pleurisy in rats by experimental suppression of the inflammatory changes. J Pathol Bacteriol. 1959 Jan;77(1):1–17. [PubMed] [Google Scholar]
- Shier W. T., DuBourdieu D. J. Polycations as prostaglandin synthesis inducers. II. Structure-activity relationships. Prostaglandins. 1986 Jun;31(6):1145–1157. doi: 10.1016/0090-6980(86)90216-9. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Dubourdieu D. J., Durkin J. P. Polycations as prostaglandin synthesis inducers. Stimulation of arachidonic acid release and prostaglandin synthesis in cultured fibroblasts by poly(L-lysine) and other synthetic polycations. Biochim Biophys Acta. 1984 Apr 18;793(2):238–250. doi: 10.1016/0005-2760(84)90326-6. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Silbert J. E., Palade G. E. Differentiated microdomains on the luminal surface of the capillary endothelium. II. Partial characterization of their anionic sites. J Cell Biol. 1981 Sep;90(3):614–621. doi: 10.1083/jcb.90.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutelsky E., Danon D. Redistribution of surface anionic sites on the luminal front of blood vessel endothelium after interaction with polycationic ligand. J Cell Biol. 1976 Oct;71(1):232–241. doi: 10.1083/jcb.71.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutelsky E., Rudich Z., Danon D. Surface charge properties of the luminal front of blood vessel walls: an electron microscopical analysis. Thromb Res. 1975 Oct;7(4):623–634. doi: 10.1016/0049-3848(75)90108-5. [DOI] [PubMed] [Google Scholar]
- Tetta C., Coda R., Camussi G. Human platelet cationic proteins bind to rat glomeruli, induce loss of anionic charges and increase glomerular permeability. Agents Actions. 1985 Mar;16(1-2):24–26. doi: 10.1007/BF01999634. [DOI] [PubMed] [Google Scholar]
- WILHELM D. L. The mediation of increased vascular permeability in inflammation. Pharmacol Rev. 1962 Jun;14:251–280. [PubMed] [Google Scholar]


