Abstract
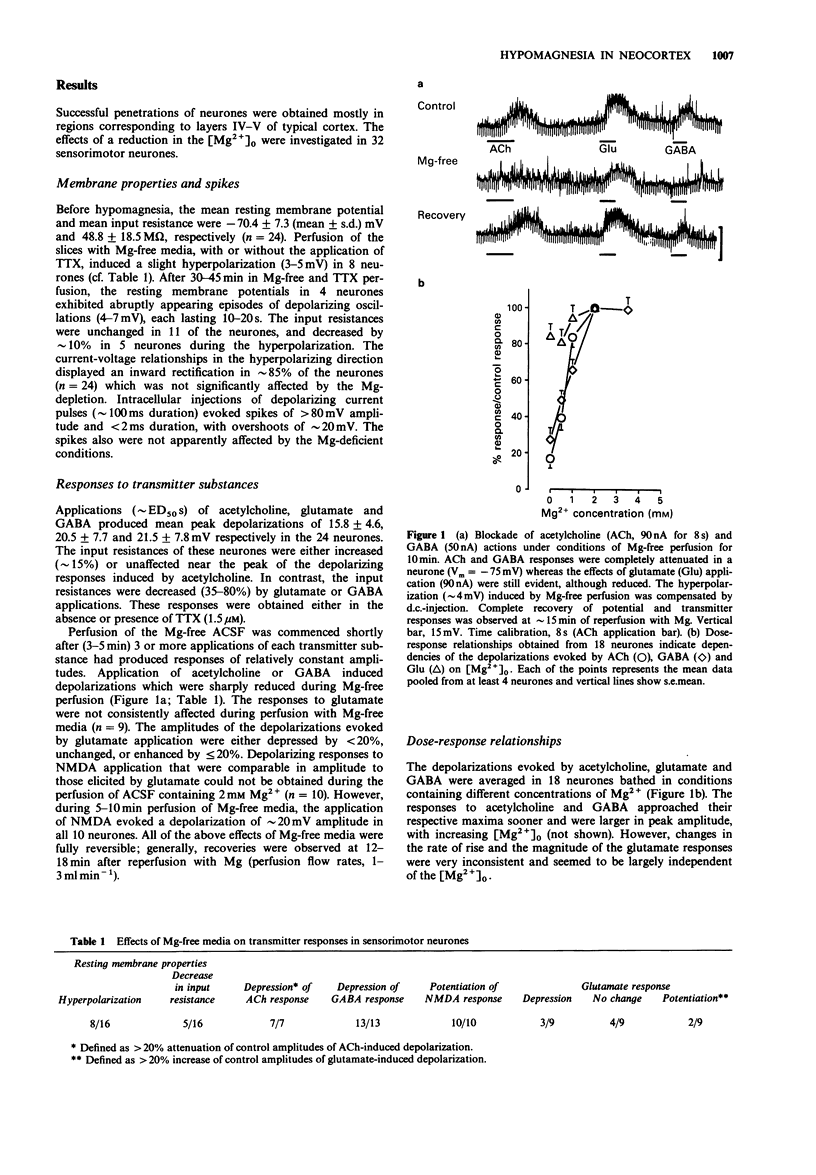
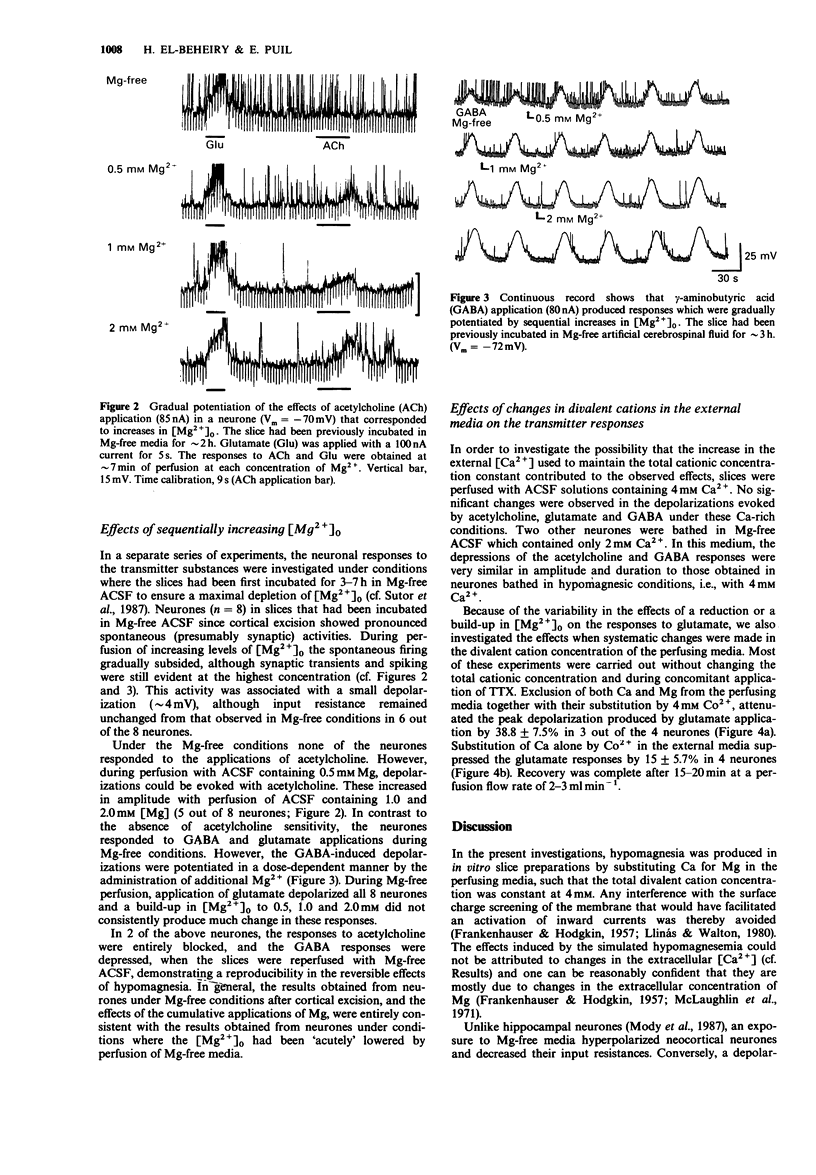
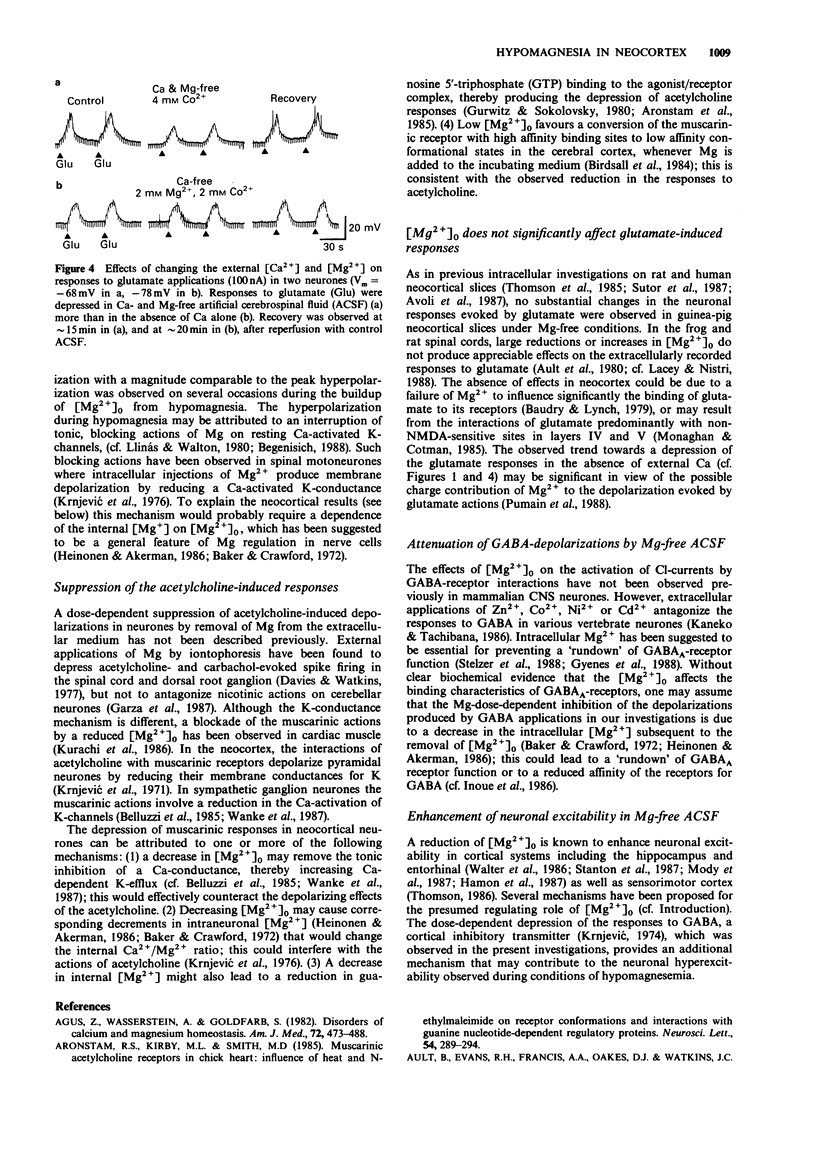
1. The effects of hypomagnesia on the neuronal responses induced by iontophorectically applied acetylcholine, glutamate, N-methylaspartate (NMDA) and gamma-aminobutyric acid (GABA) were investigated using intracellular recording techniques in in vitro slices of sensorimotor cortex (guinea-pigs). 2. Perfusion with Mg-free media, with or without tetrodotoxin (TTX), induced a small hyperpolarization (approximately 4 mV) and a small decrease (approximately 10%) in the input resistance of neurones. During TTX-blockade of Na-spike genesis, spontaneous depolarizing waves of low frequencies were observed in neurones of slices under Mg-free conditions. 3. The effects of acetylcholine and to a lesser extent, GABA actions, were depressed in a dose-dependent, reversible manner by decreases in the [Mg2+] of the perfusing media. In neurones of slices that had been incubated in Mg-free artificial cerebrospinal fluid to ensure a maximal depletion, the responses to these transmitters were potentiated by each sequentially administered increase in extracellular [Mg2+]. The actions of NMDA were potentiated during perfusion of Mg-free media. However, the responses to glutamate, which may activate receptors for NMDA, were either depressed or unchanged under these conditions. 4. A regulatory role for external Mg cations in the responses of neocortical neurones to the transmitter substances, acetylcholine and GABA, can be inferred from these investigations which simulate hypomagnesemia. The dose-dependent depression of GABA actions by low extracellular [Mg2+] additionally provides a plausible mechanism that may contribute to the neuronal hyperexcitability that is observed during conditions of hypomagnesemia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Wasserstein A., Goldfarb S. Disorders of calcium and magnesium homeostasis. Am J Med. 1982 Mar;72(3):473–488. doi: 10.1016/0002-9343(82)90519-8. [DOI] [PubMed] [Google Scholar]
- Aronstam R. S., Kirby M. L., Smith M. D. Muscarinic acetylcholine receptors in chick heart: influence of heat and N-ethylmaleimide on receptor conformations and interactions with guanine nucleotide-dependent regulatory proteins. Neurosci Lett. 1985 Mar 15;54(2-3):289–294. doi: 10.1016/s0304-3940(85)80093-8. [DOI] [PubMed] [Google Scholar]
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M., Louvel J., Pumain R., Olivier A. Seizure-like discharges induced by lowering [Mg2+]o in the human epileptogenic neocortex maintained in vitro. Brain Res. 1987 Aug 4;417(1):199–203. doi: 10.1016/0006-8993(87)90201-0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M., Lynch G. Regulation of glutamate receptors by cations. Nature. 1979 Dec 13;282(5740):748–750. doi: 10.1038/282748a0. [DOI] [PubMed] [Google Scholar]
- Begenisich T. The role of divalent cations in potassium channels. Trends Neurosci. 1988 Jun;11(6):270–273. doi: 10.1016/0166-2236(88)90109-9. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clamp. J Physiol. 1985 Jan;358:109–129. doi: 10.1113/jphysiol.1985.sp015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Czéh G., Somjen G. G. Changes in extracellular calcium and magnesium and synaptic transmission in isolated mouse spinal cord. Brain Res. 1989 May 8;486(2):274–285. doi: 10.1016/0006-8993(89)90513-1. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Effect of magnesium ions on the responses of spinal neurones to excitatory amino acids and acetylcholine. Brain Res. 1977 Jul 15;130(2):364–368. doi: 10.1016/0006-8993(77)90284-0. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs R. D., Maguire M. E. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6(3):113–127. [PubMed] [Google Scholar]
- Gurwitz D., Sokolovsky M. Agonist-specific reverse regulation of muscarinic receptors by transition metal ions and guanine nucleotides. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1296–1304. doi: 10.1016/0006-291x(80)90092-3. [DOI] [PubMed] [Google Scholar]
- Gyenes M., Farrant M., Farb D. H. "Run-down" of gamma-aminobutyric acidA receptor function during whole-cell recording: a possible role for phosphorylation. Mol Pharmacol. 1988 Dec;34(6):719–723. [PubMed] [Google Scholar]
- Hamon B., Stanton P. K., Heinemann U. An N-methyl-D-aspartate receptor-independent excitatory action of partial reduction of extracellular [Mg2+] in CA1-region of rat hippocampal slices. Neurosci Lett. 1987 Mar 31;75(2):240–245. doi: 10.1016/0304-3940(87)90304-1. [DOI] [PubMed] [Google Scholar]
- Heinonen E., Akerman K. E. Measurement of cytoplasmic, free magnesium concentration with entrapped eriochrome blue in nerve endings isolated from the guinea pig brain. Neurosci Lett. 1986 Dec 3;72(1):105–110. doi: 10.1016/0304-3940(86)90627-0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Blocking effects of cobalt and related ions on the gamma-aminobutyric acid-induced current in turtle retinal cones. J Physiol. 1986 Apr;373:463–479. doi: 10.1113/jphysiol.1986.sp016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. Intracellular Mg2+ increases neuronal excitability. Can J Physiol Pharmacol. 1976 Feb;54(1):73–77. doi: 10.1139/y76-012. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Role of intracellular Mg2+ in the activation of muscarinic K+ channel in cardiac atrial cell membrane. Pflugers Arch. 1986 Nov;407(5):572–574. doi: 10.1007/BF00657521. [DOI] [PubMed] [Google Scholar]
- Lacey G., Nistri A. Changes in glutamate-evoked currents of frog motoneurones by extracellular Mg2+. Neurosci Lett. 1988 Jul 19;90(1-2):213–218. doi: 10.1016/0304-3940(88)90814-2. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Miljkovic Z., Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987 Aug;58(2):251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Martindale L., Heaton F. W. Magnesium deficiency in the adult rat. Biochem J. 1964 Jul;92(1):119–126. doi: 10.1042/bj0920119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I., Lambert J. D., Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987 Mar;57(3):869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P. K., Jones R. S., Mody I., Heinemann U. Epileptiform activity induced by lowering extracellular [Mg2+] in combined hippocampal-entorhinal cortex slices: modulation by receptors for norepinephrine and N-methyl-D-aspartate. Epilepsy Res. 1987 Jan;1(1):53–62. doi: 10.1016/0920-1211(87)90051-9. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Sutor B., Jordan W., Zieglgänsberger W. Evidence for a magnesium-insensitive membrane resistance increase during NMDA-induced depolarizations in rat neocortical neurons in vitro. Neurosci Lett. 1987 Apr 10;75(3):317–322. doi: 10.1016/0304-3940(87)90542-8. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. A magnesium-sensitive post-synaptic potential in rat cerebral cortex resembles neuronal responses to N-methylaspartate. J Physiol. 1986 Jan;370:531–549. doi: 10.1113/jphysiol.1986.sp015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985 Feb 7;313(6002):479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Walther H., Lambert J. D., Jones R. S., Heinemann U., Hamon B. Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett. 1986 Aug 29;69(2):156–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- Wanke E., Ferroni A., Malgaroli A., Ambrosini A., Pozzan T., Meldolesi J. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Garza R., McGuire T. J., Freedman R., Hoffer B. J. The electrophysiological effects of nicotine in the rat cerebellum: evidence for direct postsynaptic actions. Neurosci Lett. 1987 Oct 5;80(3):303–308. doi: 10.1016/0304-3940(87)90472-1. [DOI] [PubMed] [Google Scholar]
- el-Beheiry H., Puil E. Postsynaptic depression induced by isoflurane and Althesin in neocortical neurons. Exp Brain Res. 1989;75(2):361–368. doi: 10.1007/BF00247942. [DOI] [PubMed] [Google Scholar]


