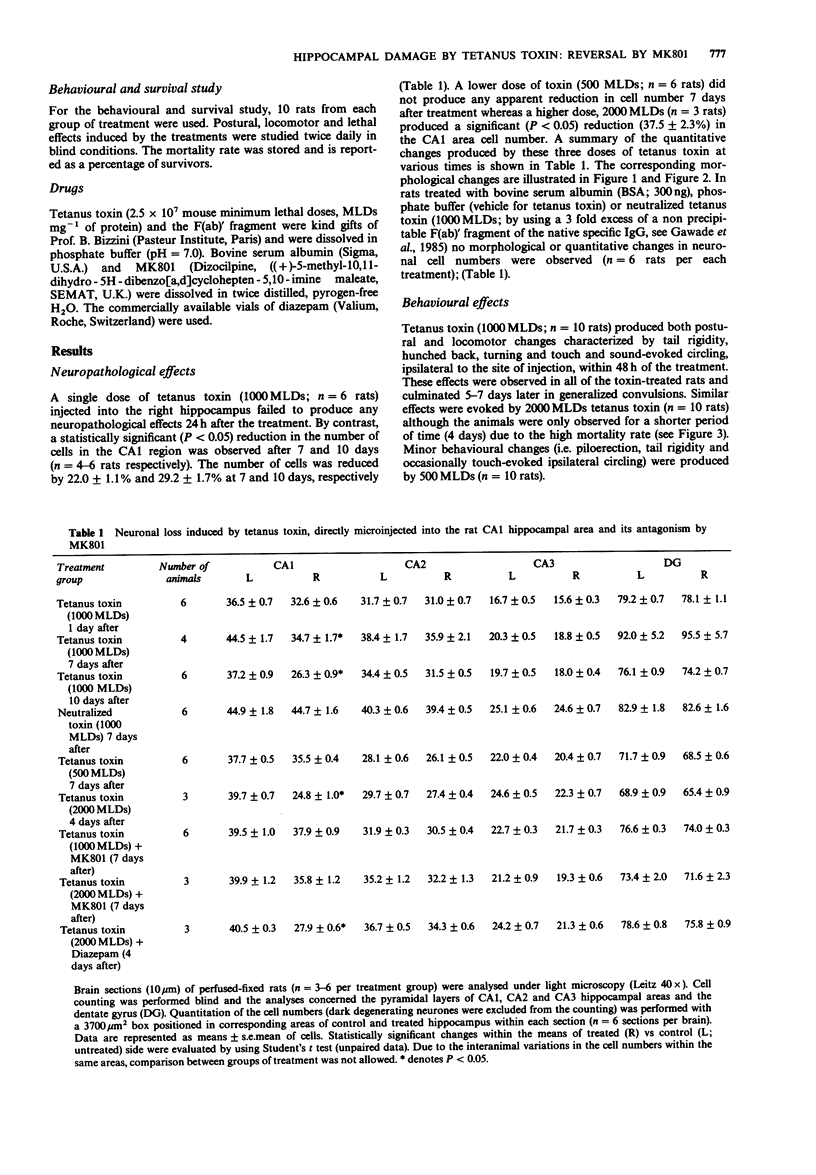
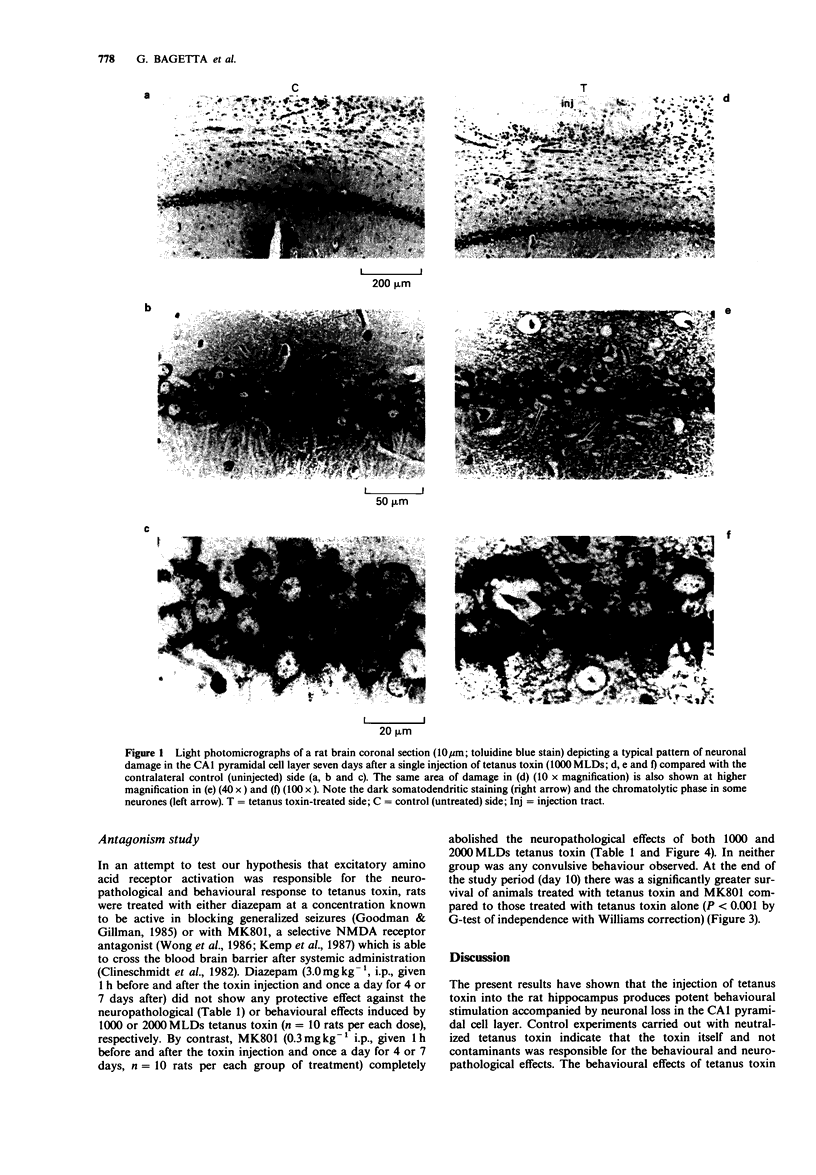
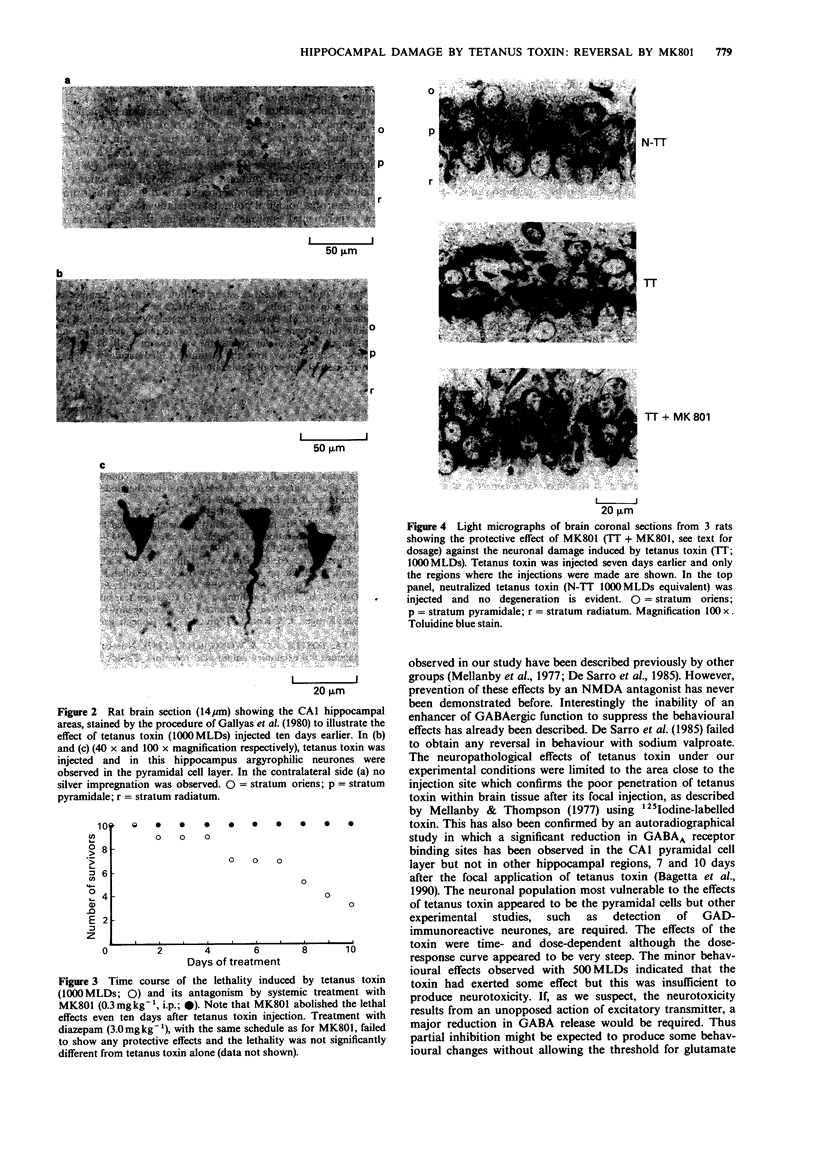
Abstract
1. The behavioural and neuropathological effects of tetanus toxin, microinjected directly into the hippocampus, were studied in rats. 2. A single dose (1000 minimum lethal doses, MLDs) of tetanus toxin, injected unilaterally into the hippocampus produced a time-dependent neuronal loss in the CA1 pyramidal cell layer. In comparison with the contralateral, untreated side these effects became statistically significant (P less than 0.05) 7 days (22.0 +/- 1.1% reduction) and 10 days (29.2 +/- 1.7% reduction) after the injection. No significant changes were observed 7 days after treatment with 500 MLDs whereas a reduction of 37.5 +/- 3.1% in the CA1 area cell number was produced 4 days after the injection of 2000 MLDs. 3. Behavioral stimulatory effects were also induced by tetanus toxin (1000 MLDs) within 48 h of the injection and these culminated in generalized convulsions 5-7 days later. Convulsions were observed after a shorter period of latency in rats receiving 2000 MLDs tetanus toxin whereas 500 MLDs were ineffective. 4. No behavioural and neuropathological effects were observed in rats treated with neutralized tetanus toxin (1000 MLDs), bovine serum albumin or phosphate buffer. 5. Pretreatment with MK801 (0.3 mg kg-1, i.p., given 1 h before and after the injection with tetanus toxin and then once daily for 4 or 7 days) prevented the behavioural and neuropathological effects induced by tetanus toxin (1000-2000 MLDs). In addition, such treatment fully protected the animals from the lethal effects induced by 1000 MLDs tetanus toxin.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagetta G., Knott C., Nisticó G., Bowery N. G. Tetanus toxin produces neuronal loss and a reduction in GABAA but not GABAB binding sites in rat hippocampus. Neurosci Lett. 1990 Feb 5;109(1-2):7–12. doi: 10.1016/0304-3940(90)90529-i. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Drejer J., Schousboe A., Diemer N. H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984 Nov;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bergey G. K., Bigalke H., Nelson P. G. Differential effects of tetanus toxin on inhibitory and excitatory synaptic transmission in mammalian spinal cord neurons in culture: a presynaptic locus of action for tetanus toxin. J Neurophysiol. 1987 Jan;57(1):121–131. doi: 10.1152/jn.1987.57.1.121. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Benedetti M., Mercuri N. B., Bernardi G. Selective depression of synaptic transmission by tetanus toxin: a comparative study on hippocampal and neostriatal slices. Neuroscience. 1989;30(3):663–670. doi: 10.1016/0306-4522(89)90159-0. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Thompson P. A., Davies J., Mellanby J. In vitro effect of tetanus toxin on GABA release form rat hippocampal slices. J Neurochem. 1981 Oct;37(4):1039–1041. doi: 10.1111/j.1471-4159.1981.tb04492.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., De Groat W. C. Tetanus toxin and spinal inhibition. Brain Res. 1968 Aug 26;10(2):208–212. doi: 10.1016/0006-8993(68)90123-6. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Felix D., Game C. J., McCulloch R. M. Tetanus toxin and the synaptic release of GABA. Brain Res. 1973 Mar 15;51:358–362. doi: 10.1016/0006-8993(73)90389-2. [DOI] [PubMed] [Google Scholar]
- Davies J., Tongroach P. Tetanus toxin and synaptic inhibition in the substantia nigra and striatum of the rat. J Physiol. 1979 May;290(2):23–36. doi: 10.1113/jphysiol.1979.sp012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg G. E., Foster A. C. Amino acid neurotransmitters and their pathways in the mammalian central nervous system. Neuroscience. 1983 Aug;9(4):701–719. doi: 10.1016/0306-4522(83)90263-4. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Gill R., Kemp J. A., Woodruff G. N. Systemic administration of MK-801 prevents N-methyl-D-aspartate-induced neuronal degeneration in rat brain. Neurosci Lett. 1987 May 19;76(3):307–311. doi: 10.1016/0304-3940(87)90420-4. [DOI] [PubMed] [Google Scholar]
- Gallyas F., Wolff J. R., Böttcher H., Zaborszky L. A reliable and sensitive method to localize terminal degeneration and lysosomes in the central nervous system. Stain Technol. 1980 Sep;55(5):299–306. doi: 10.3109/10520298009067258. [DOI] [PubMed] [Google Scholar]
- Gawade S., Bon C., Bizzini B. The use of antibody Fab fragments specifically directed to two different complementary parts of the tetanus toxin molecule for studying the mode of action of the toxin. Brain Res. 1985 May 13;334(1):139–146. doi: 10.1016/0006-8993(85)90575-x. [DOI] [PubMed] [Google Scholar]
- Gill R., Foster A. C., Woodruff G. N. Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. J Neurosci. 1987 Oct;7(10):3343–3349. doi: 10.1523/JNEUROSCI.07-10-03343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby J., George G., Robinson A., Thompson P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J Neurol Neurosurg Psychiatry. 1977 Apr;40(4):404–414. doi: 10.1136/jnnp.40.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981 Jul;37(1):1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Suzuki R., Yamaguchi T., Li C. L., Klatzo I. The effects of 5-minute ischemia in Mongolian gerbils: II. Changes of spontaneous neuronal activity in cerebral cortex and CA1 sector of hippocampus. Acta Neuropathol. 1983;60(3-4):217–222. doi: 10.1007/BF00691869. [DOI] [PubMed] [Google Scholar]
- Taylor C. P. How do seizures begin? Clues from hippocampal slices. Trends Neurosci. 1988 Sep;11(9):375–378. doi: 10.1016/0166-2236(88)90070-7. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]