Abstract
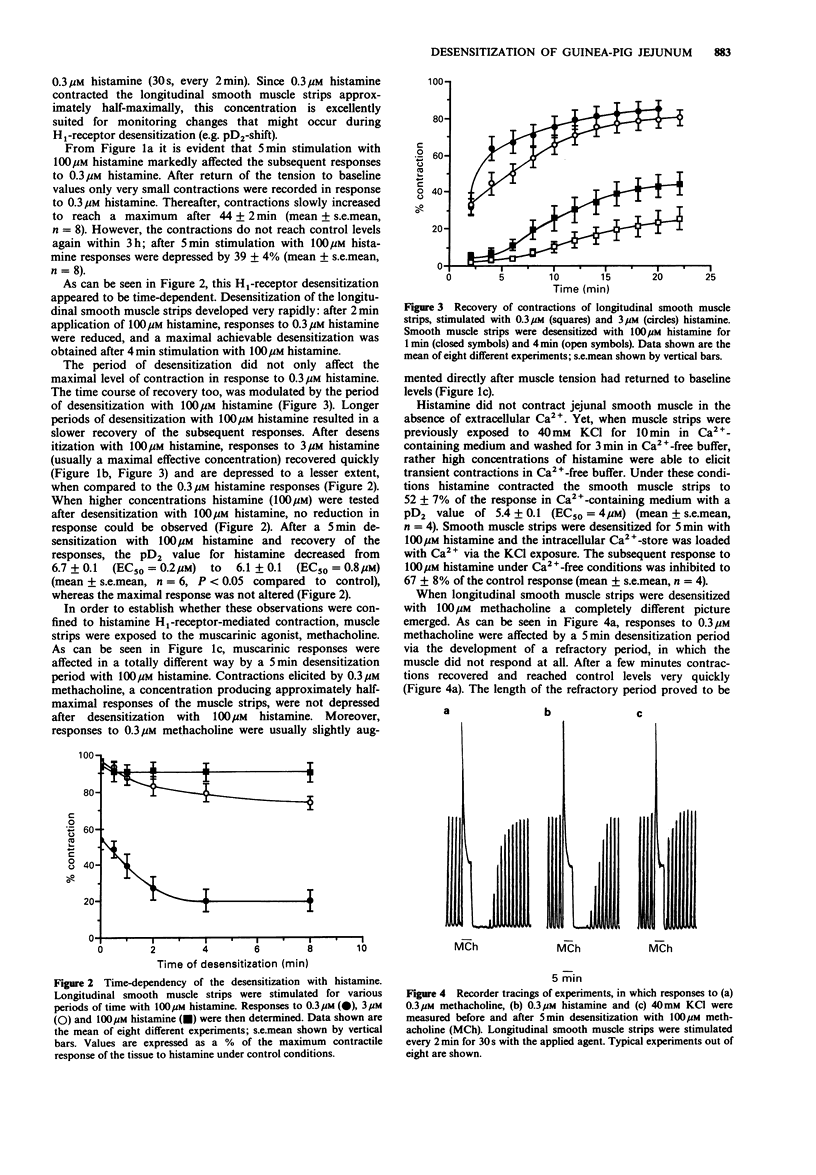
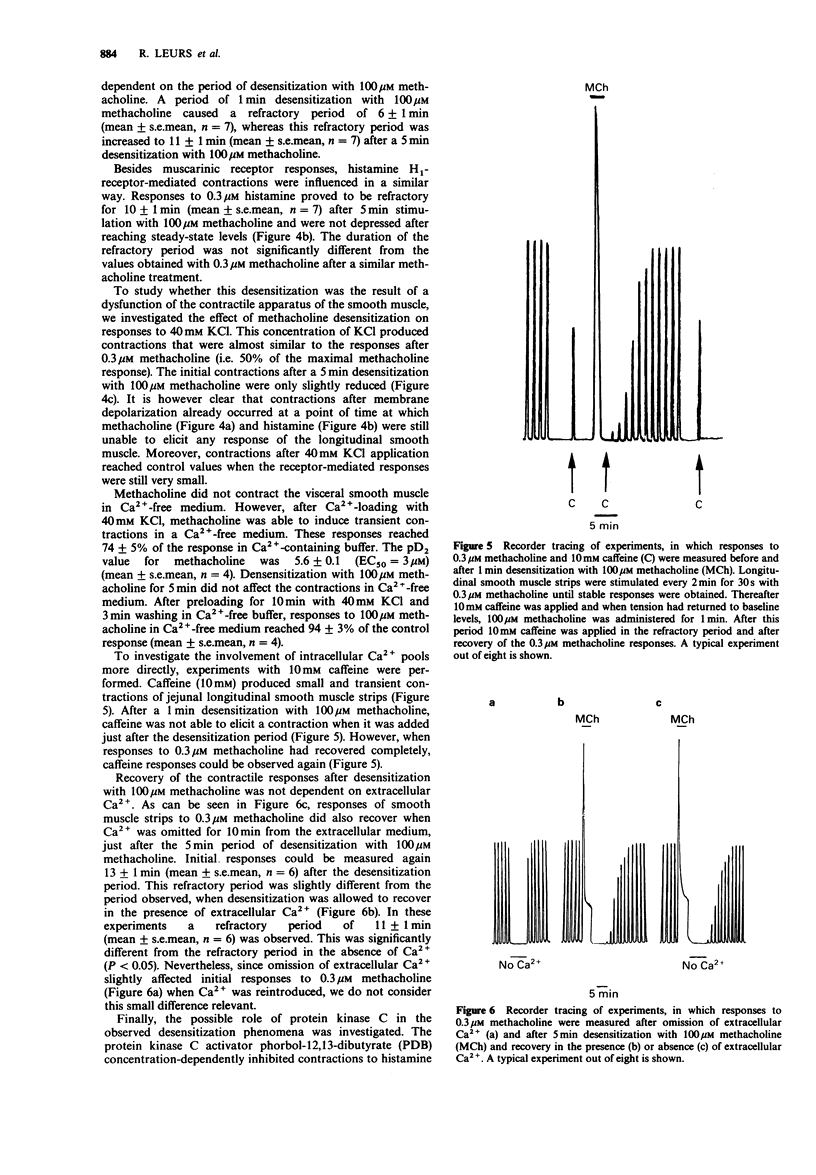
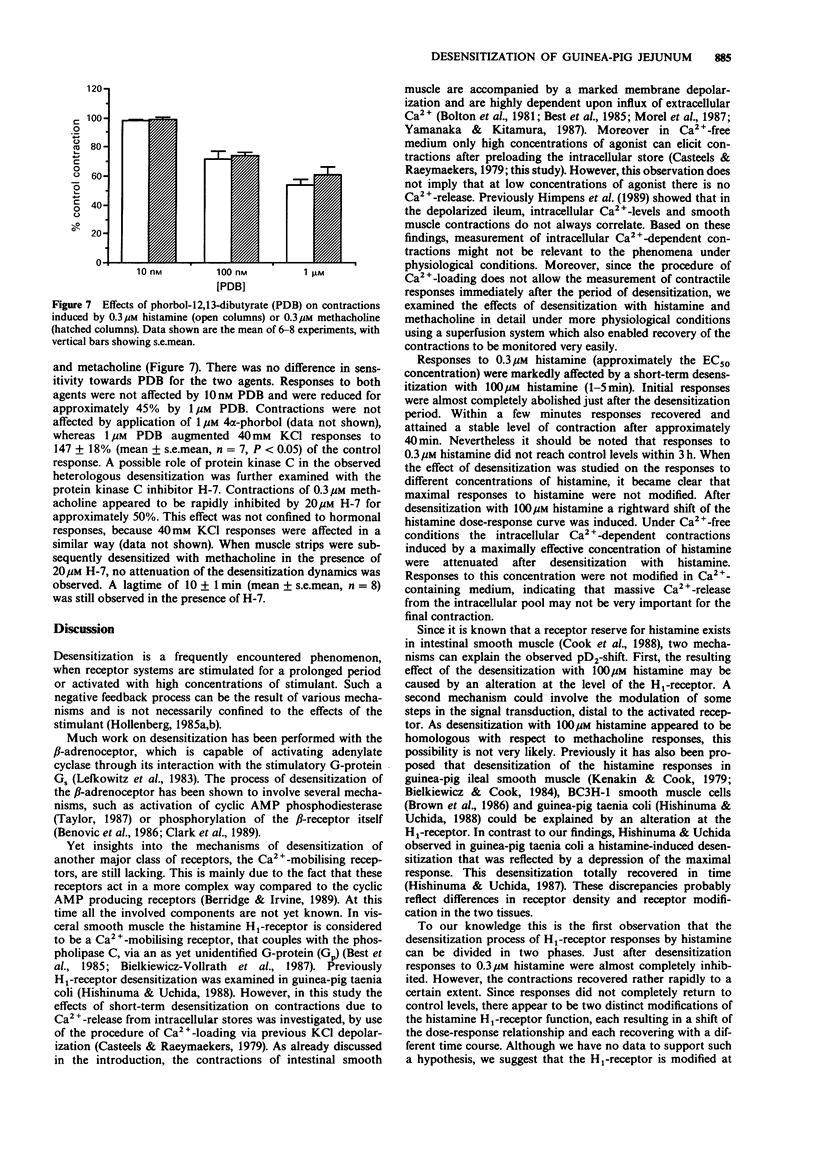
1. In the present study we investigated desensitization phenomena of guinea-pig jejunal longitudinal smooth muscle responses after stimulation with 100 microM histamine or methacholine, using a superfusion method. 2. Histamine H1-receptor-mediated contractions appear to be rapidly reduced after application of 100 microM histamine. Muscarinic responses were not affected following desensitization with 100 microM histamine, indicating a homologous desensitization. 3. Initial contractions to 0.3 microM histamine were reduced by 90%, recovered quickly, but did not reach control levels within 1 h. Desensitization of histamine responses could be separated into two phases; a rapid, but transient, desensitization and a more sustained desensitization. As a consequence of this sustained effect the pD2 for histamine shifted from 6.7 +/- 0.1 (control) to 6.1 +/- 0.1 (desensitized). 4. Desensitization with 100 microM methacholine caused a heterologous desensitization, reflected by the development of a refractory period, in which neither histamine nor methacholine was able to elicit a contraction. After a few minutes responses to both agents recovered to control levels. 5. During the refractory period after methacholine desensitization, muscle strips were still responsive to 40 mM KCl but did not contract in response to 10 mM caffeine, suggesting that the heterologous desensitization is caused by a modification of an intracellular Ca2(+)-store, which is used by both histamine and methacholine. 6. The recovery of the responses after methacholine desensitization was not dependent on extracellular Ca2+, suggesting that the recovery is not dependent on refilling of the intracellular Ca2+ store with extracellular Ca2+. 7. The protein kinase C activator, phorbol-12,13-dibutyrate, concentration-dependently inhibited histamine- and methacholine-induced contractions.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. H., Krzanowski J. J., Polson J. B., Szentivanyi A. Characteristics of histamine tachyphylaxis in canine tracheal smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1979 Aug;308(2):117–125. doi: 10.1007/BF00499053. [DOI] [PubMed] [Google Scholar]
- Baenziger N. L., Fogerty F. J., Mertz L. F., Chernuta L. F. Regulation of histamine-mediated prostacyclin synthesis in cultured human vascular endothelial cells. Cell. 1981 Jun;24(3):915–923. doi: 10.1016/0092-8674(81)90117-3. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., DeBlasi A., Stone W. C., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989 Oct 13;246(4927):235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Best L., Brooks K. J., Bolton T. B. Relationship between stimulated inositol lipid hydrolysis and contractility in guinea-pig visceral longitudinal smooth muscle. Biochem Pharmacol. 1985 Jul 1;34(13):2297–2301. doi: 10.1016/0006-2952(85)90785-3. [DOI] [PubMed] [Google Scholar]
- Bielkiewicz-Vollrath B., Carpenter J. R., Schulz R., Cook D. A. Early production of 1,4,5-inositol trisphosphate and 1,3,4,5-inositol tetrakisphosphate by histamine and carbachol in ileal smooth muscle. Mol Pharmacol. 1987 May;31(5):513–522. [PubMed] [Google Scholar]
- Bielkiewicz B., Cook D. A. The mechanism of the histamine-induced desensitization of guinea-pig ileum. Gen Pharmacol. 1984;15(1):51–54. doi: 10.1016/0306-3623(84)90080-6. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P., Kitamura K., Lang R. J. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981 Nov;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. D., Prendiville P., Cain C. Alpha 1-adrenergic and H1-histamine receptor control of intracellular Ca2+ in a muscle cell line: the influence of prior agonist exposure on receptor responsiveness. Mol Pharmacol. 1986 Jun;29(6):531–539. [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Friedman J., Dixon R. A., Strader C. D. Identification of a specific site required for rapid heterologous desensitization of the beta-adrenergic receptor by cAMP-dependent protein kinase. Mol Pharmacol. 1989 Sep;36(3):343–348. [PubMed] [Google Scholar]
- Cook D. A., Yong M. S., Ramji K., Vollrath B. The action of agonists and antagonists at the histamine H1 receptor and receptor protection studies in guinea pig ileum. Can J Physiol Pharmacol. 1988 May;66(5):618–623. doi: 10.1139/y88-096. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. 1,4-Dithiothreitol-induced changes in histamine H1-agonist efficacy and affinity in the longitudinal smooth muscle of guinea-pig ileum. Br J Pharmacol. 1987 Jan;90(1):263–271. doi: 10.1111/j.1476-5381.1987.tb16848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens B., Casteels R. Measurement by Quin2 of changes of the intracellular calcium concentration in strips of the rabbit ear artery and of the guinea-pig ileum. Pflugers Arch. 1987 Jan;408(1):32–37. doi: 10.1007/BF00581837. [DOI] [PubMed] [Google Scholar]
- Himpens B., Matthijs G., Somlyo A. P. Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J Physiol. 1989 Jun;413:489–503. doi: 10.1113/jphysiol.1989.sp017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma S., Uchida M. K. Effects of local anaesthetics on short-term desensitization of guinea-pig taenia caecum to histamine. Br J Pharmacol. 1987 Dec;92(4):733–741. doi: 10.1111/j.1476-5381.1987.tb11377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma S., Uchida M. K. Short-term desensitization of guinea-pig taenia caecum induced by carbachol occurs at intracellular Ca stores and that by histamine at H1-receptors. Br J Pharmacol. 1988 Jul;94(3):882–889. doi: 10.1111/j.1476-5381.1988.tb11600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Weiss G. B. Calcium release in smooth muscle. Life Sci. 1988;42(2):111–122. doi: 10.1016/0024-3205(88)90674-1. [DOI] [PubMed] [Google Scholar]
- Kenakin T. P., Cook D. A. The effect of desensitization on the antagonism of the histamine response by phenoxybenzamine. Mol Pharmacol. 1980 May;17(3):309–313. [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., DeBlasi A., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. I. Agonist-promoted desensitization and phosphorylation of alpha 1-adrenergic receptors coupled to inositol phospholipid metabolism in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1987 Mar 5;262(7):3098–3105. [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Leurs R., Bast A., Timmerman H. Essential thiol and disulphide groups in the histamine H1-receptor signal transfer of guinea-pig parenchymal lung strips. Agents Actions. 1990 Apr;30(1-2):169–173. doi: 10.1007/BF01969029. [DOI] [PubMed] [Google Scholar]
- Manning P. J., Jones G. L., O'Byrne P. M. Tachyphylaxis to inhaled histamine in asthmatic subjects. J Appl Physiol (1985) 1987 Oct;63(4):1572–1577. doi: 10.1152/jappl.1987.63.4.1572. [DOI] [PubMed] [Google Scholar]
- McDonough P. M., Eubanks J. H., Brown J. H. Desensitization and recovery of muscarinic and histaminergic Ca2+ mobilization in 1321N1 astrocytoma cells. Biochem J. 1988 Jan 1;249(1):135–141. doi: 10.1042/bj2490135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N., Hardy J. P., Godfraind T. Histamine-operated calcium channels in intestinal smooth muscle of the guinea-pig. Eur J Pharmacol. 1987 Mar 3;135(1):69–75. doi: 10.1016/0014-2999(87)90758-8. [DOI] [PubMed] [Google Scholar]
- Nakahata N., Harden T. K. Regulation of inositol trisphosphate accumulation by muscarinic cholinergic and H1-histamine receptors on human astrocytoma cells. Differential induction of desensitization by agonists. Biochem J. 1987 Jan 15;241(2):337–344. doi: 10.1042/bj2410337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P., Dettbarn C., Brunder D., Stein P., Hals G. Pharmacology of calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989 Apr;21(2):295–320. doi: 10.1007/BF00812074. [DOI] [PubMed] [Google Scholar]
- Taylor S. E. Potentiation of isoproterenol-induced relaxation of isolated trachea by aminophylline: modulation by desensitization. Proc Soc Exp Biol Med. 1987 Sep;185(4):385–391. doi: 10.3181/00379727-185-42558. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Stanley A. F., Sasaguri T. Does the hydrolysis of inositol phospholipids lead to the opening of voltage operated Ca2+ channels in guinea-pig ileum? Studies with fluoride ions and caffeine. Biochem Biophys Res Commun. 1988 May 31;153(1):14–20. doi: 10.1016/s0006-291x(88)81183-5. [DOI] [PubMed] [Google Scholar]
- Yamanaka K., Kitamura K. Electrophysiological and mechanical characteristics of histamine receptors in smooth muscle cells of the guinea-pig ileum. Eur J Pharmacol. 1987 Nov 24;144(1):29–37. doi: 10.1016/0014-2999(87)90005-7. [DOI] [PubMed] [Google Scholar]


