Abstract
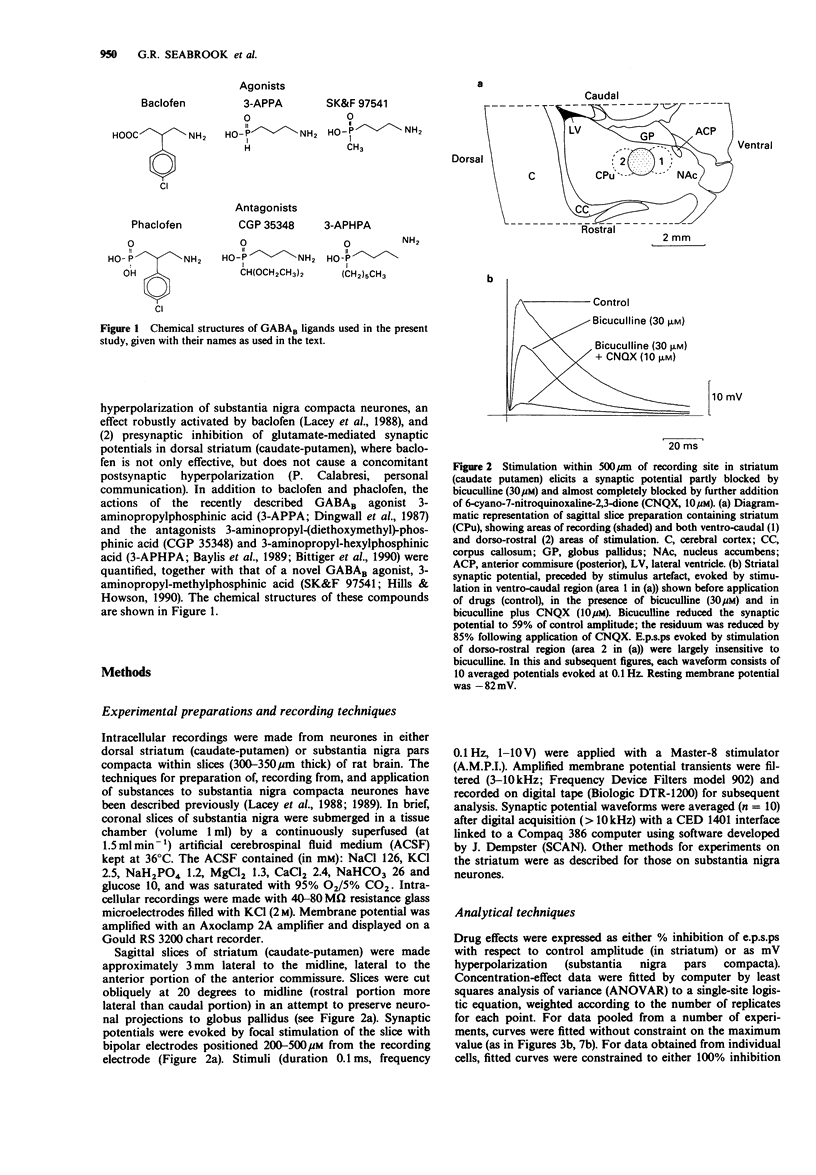
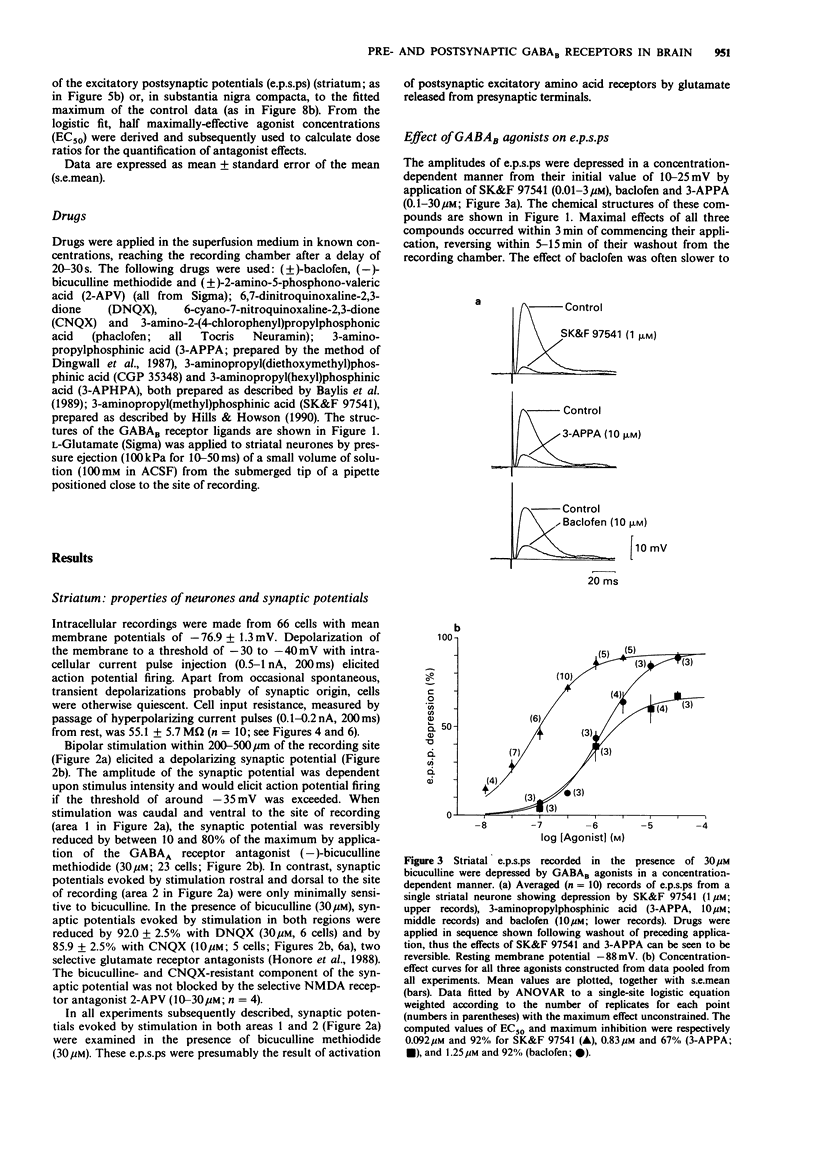
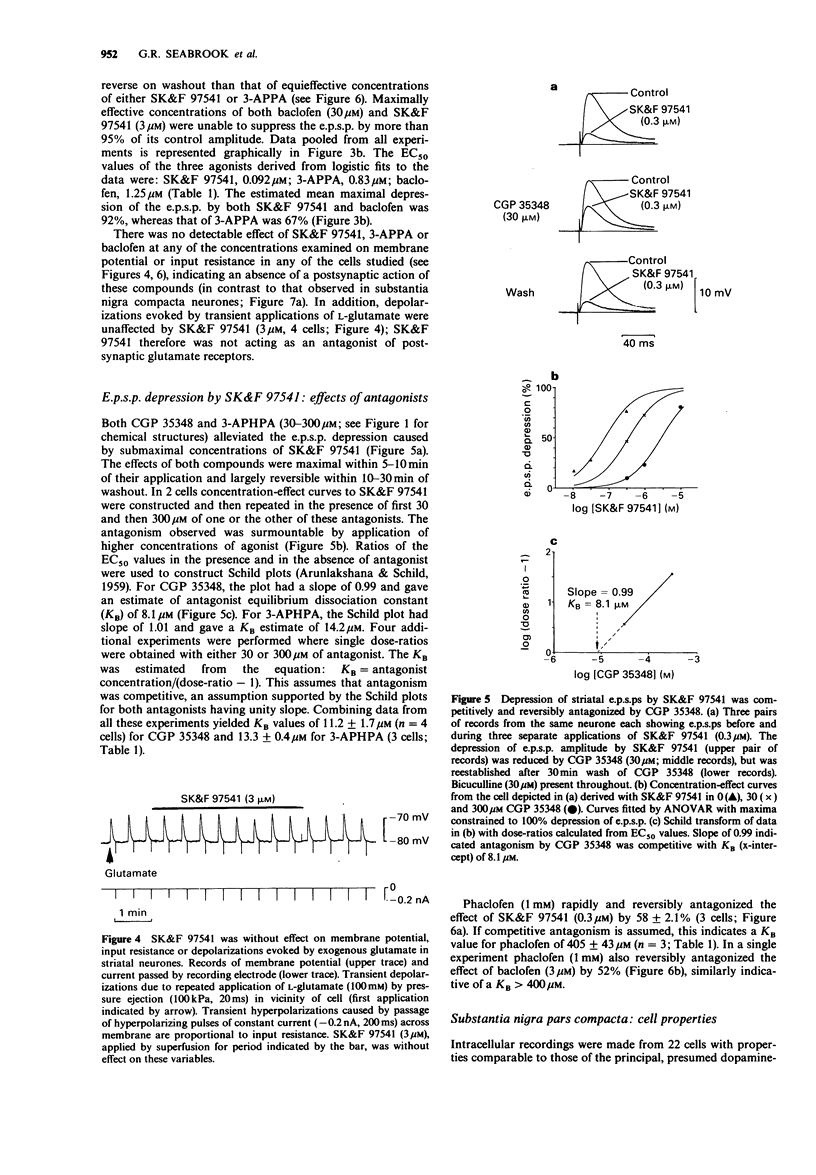
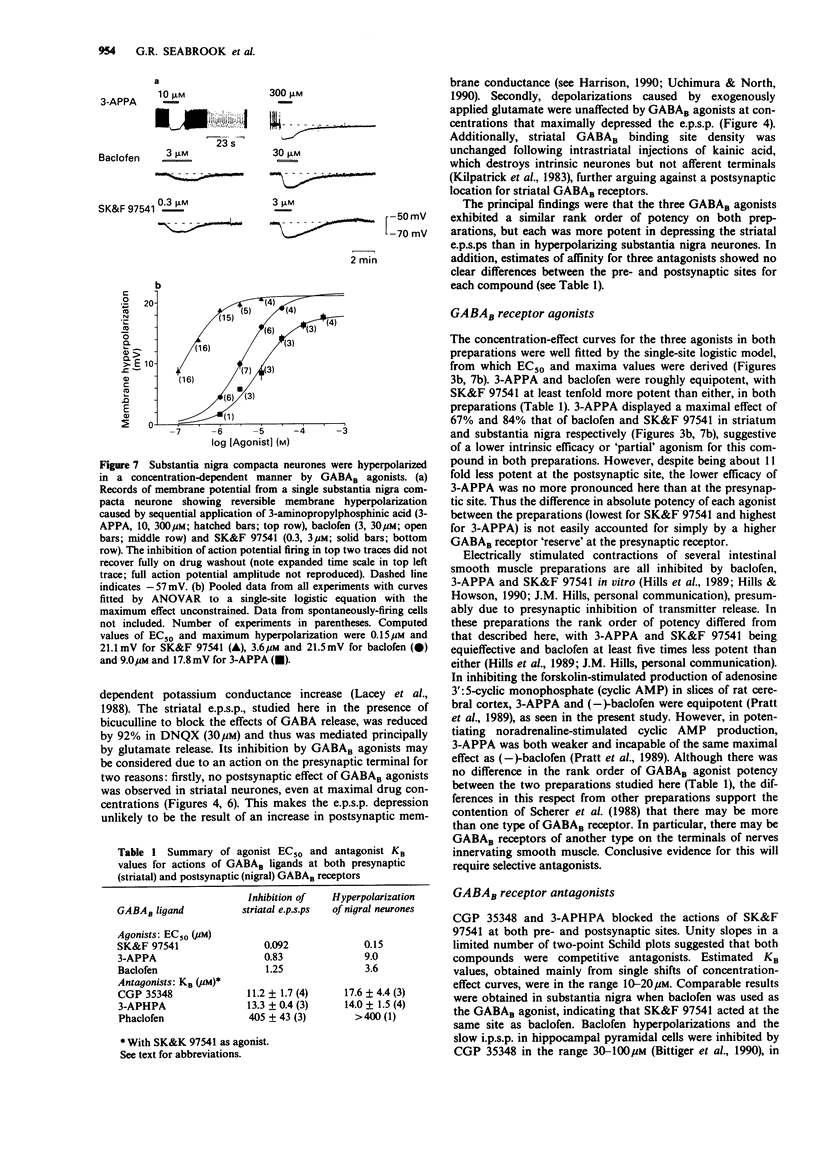
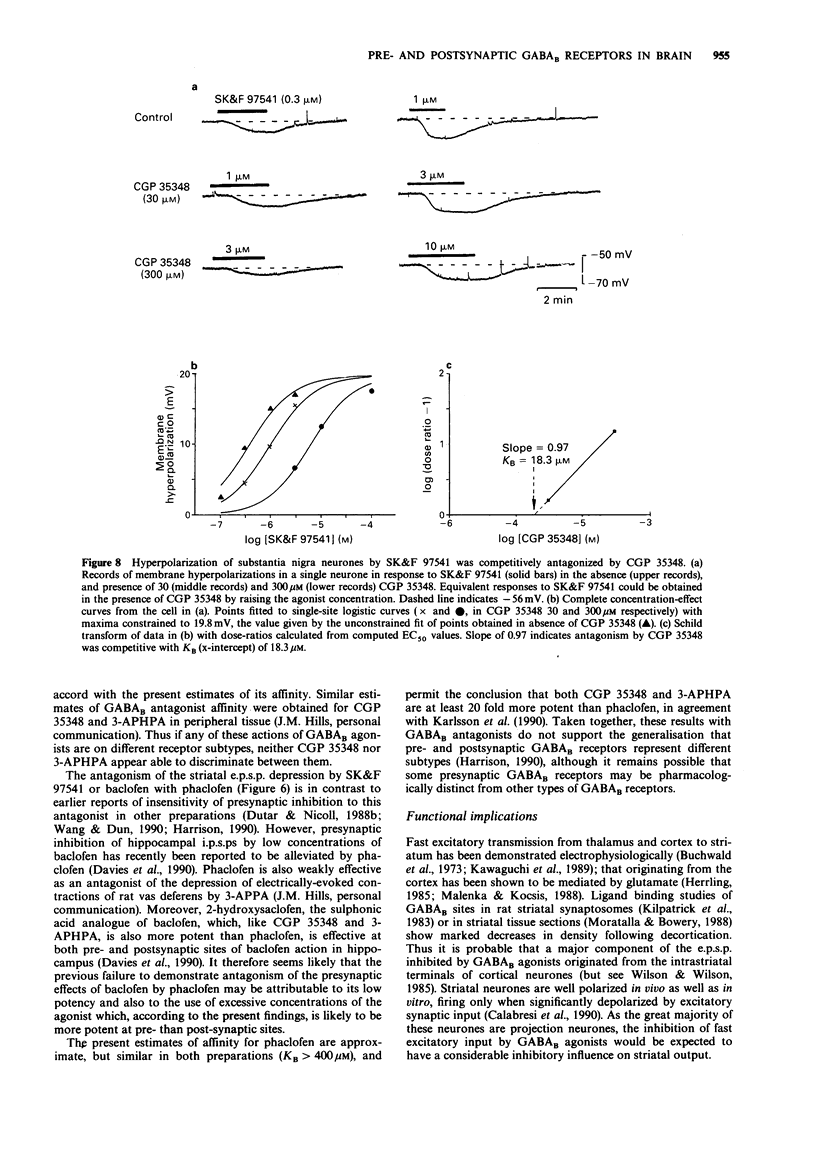
1. Intracellular recordings were made from neurons in striatum (caudate-putamen) and substantia nigra pars compacta in rat brain slices. Three GABAB agonists, baclofen, 3-aminopropylphosphinic acid (3-APPA) and 3-aminopropyl(methyl)phosphinic acid (SK&F 97541), depressed excitatory postsynaptic potentials (e.p.s.ps) mediated by glutamate in the striatum, and hyperpolarized neurones in the substantia nigra. The ability of 3-aminopropyl(diethyoxymethyl)phosphinic acid (CGP 35348), 3-aminopropyl (hexyl)phosphinic acid (3-APHPA) and phaclofen to antagonize these responses was assessed. 2. Striatal e.p.s.ps, studied in the presence of bicuculline (30 microns), were reduced in amplitude by 92% with 6,7-dinitroquinoxaline-2,3-dione (DNQX; 30 microns). These e.p.s.ps were depressed by up to 95% by SK&F 97541 and baclofen with EC50s of 0.092 microns and 1.25 microns respectively. The maximal effect of 3-APPA was 67% with an EC50 of 0.83 microns. Agonist concentration-effect data fitted a single-site logistic model. GABAB agonists were without effect on striatal neurone membrane potential, input resistance or depolarizations induced by applied glutamate. 3. The depression of striatal e.p.s.ps by SK&F 97541 was reversibly antagonized by CGP 35348, 3-APHPA and phaclofen with estimated equilibrium dissociation constants (KB) of 11.2 +/- 1.7 microns (n = 4), 13.3 +/- 0.4 microM (n = 3) and 405 +/- 43 microM (n = 3) respectively. CGP 35348 and 3-APHPA appeared to act competitively (Schild plot slopes of 0.99 and 1.01 respectively). 4. Nigral neurones were hyperpolarized by up to 25 mV by SK&F 97541 and baclofen with EC50s of 0.15 microns and 3.6 microns respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin R. L., Young A. B., Penney J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989 Oct;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987 Feb;20(2):365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989 Oct;10(10):401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Buchwald N. A., Price D. D., Vernon L., Hull C. D. Caudate intracellular response to thalamic and cortical inputs. Exp Neurol. 1973 Feb;38(2):311–323. doi: 10.1016/0014-4886(73)90155-6. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N. B., Stefani A., Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons. I. An in vivo analysis. J Neurophysiol. 1990 Apr;63(4):651–662. doi: 10.1152/jn.1990.63.4.651. [DOI] [PubMed] [Google Scholar]
- Chu D. C., Albin R. L., Young A. B., Penney J. B. Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience. 1990;34(2):341–357. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- Colmers W. F., Williams J. T. Pertussis toxin pretreatment discriminates between pre- and postsynaptic actions of baclofen in rat dorsal raphe nucleus in vitro. Neurosci Lett. 1988 Nov 11;93(2-3):300–306. doi: 10.1016/0304-3940(88)90099-7. [DOI] [PubMed] [Google Scholar]
- Cordingley G. E., Weight F. F. Non-cholinergic synaptic excitation in neostriatum: pharmacological evidence for mediation by a glutamate-like transmitter. Br J Pharmacol. 1986 Aug;88(4):847–856. doi: 10.1111/j.1476-5381.1986.tb16258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Harrison N. L. On the presynaptic action of baclofen at inhibitory synapses between cultured rat hippocampal neurones. J Physiol. 1990 Mar;422:433–446. doi: 10.1113/jphysiol.1990.sp017993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasuo H., Gallagher J. P. Comparison of antagonism by phaclofen of baclofen induced hyperpolarizations and synaptically mediated late hyperpolarizing potentials recorded intracellularly from rat dorsolateral septal neurons. Neurosci Lett. 1988 Mar 21;86(1):77–81. doi: 10.1016/0304-3940(88)90186-3. [DOI] [PubMed] [Google Scholar]
- Herrling P. L. Pharmacology of the corticocaudate excitatory postsynaptic potential in the cat: evidence for its mediation by quisqualate- or kainate-receptors. Neuroscience. 1985 Feb;14(2):417–426. doi: 10.1016/0306-4522(85)90301-x. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Hills J. M., Dingsdale R. A., Parsons M. E., Dolle R. E., Howson W. 3-Aminopropylphosphinic acid--a potent, selective GABAB receptor agonist in the guinea-pig ileum and rat anococcygeus muscle. Br J Pharmacol. 1989 Aug;97(4):1292–1296. doi: 10.1111/j.1476-5381.1989.tb12591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Wilson C. J., Emson P. C. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989 Nov;62(5):1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988 Jul;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989 Apr;9(4):1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Kocsis J. D. Presynaptic actions of carbachol and adenosine on corticostriatal synaptic transmission studied in vitro. J Neurosci. 1988 Oct;8(10):3750–3756. doi: 10.1523/JNEUROSCI.08-10-03750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989 Nov;62(5):1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Scherer R. W., Ferkany J. W., Enna S. J. Evidence for pharmacologically distinct subsets of GABAB receptors. Brain Res Bull. 1988 Sep;21(3):439–443. doi: 10.1016/0361-9230(88)90156-6. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Haby M., Leresche N., Crunelli V. The GABAB antagonist phaclofen inhibits the late K+-dependent IPSP in cat and rat thalamic and hippocampal neurones. Brain Res. 1988 May 17;448(2):351–354. doi: 10.1016/0006-8993(88)91275-9. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H. Evidence that guanosine triphosphate (GTP)-binding proteins control a synaptic response in brain: effect of pertussis toxin and GTP gamma S on the late inhibitory postsynaptic potential of hippocampal CA3 neurons. J Neurosci. 1988 Dec;8(12):4589–4602. doi: 10.1523/JNEUROSCI.08-12-04589.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. Y., Dun N. J. Phaclofen-insensitive presynaptic inhibitory action of (+/-)-baclofen in neonatal rat motoneurones in vitro. Br J Pharmacol. 1990 Feb;99(2):413–421. doi: 10.1111/j.1476-5381.1990.tb14718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. S., Wilson J. A. Baclofen attenuates hyperpolarizing not depolarizing responses of caudate neurons in cat. Brain Res. 1985 Sep 9;342(2):396–400. doi: 10.1016/0006-8993(85)91145-x. [DOI] [PubMed] [Google Scholar]


