Abstract
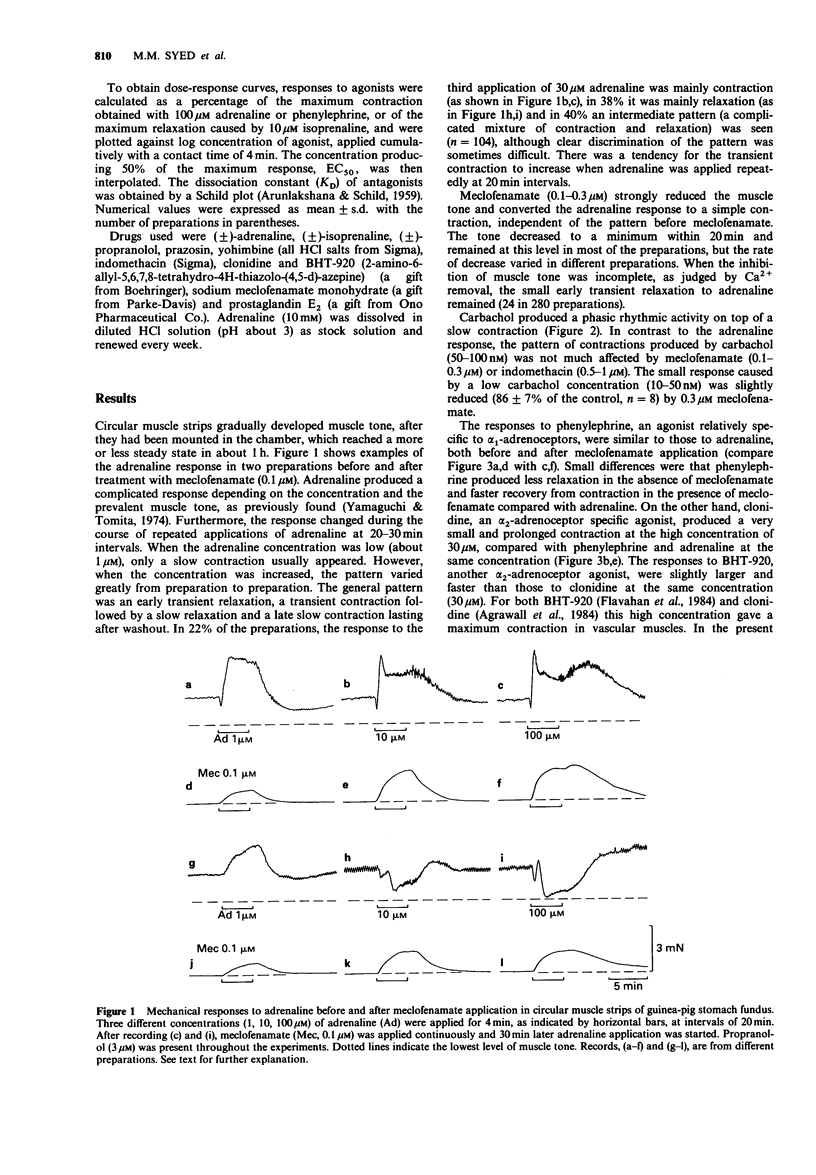
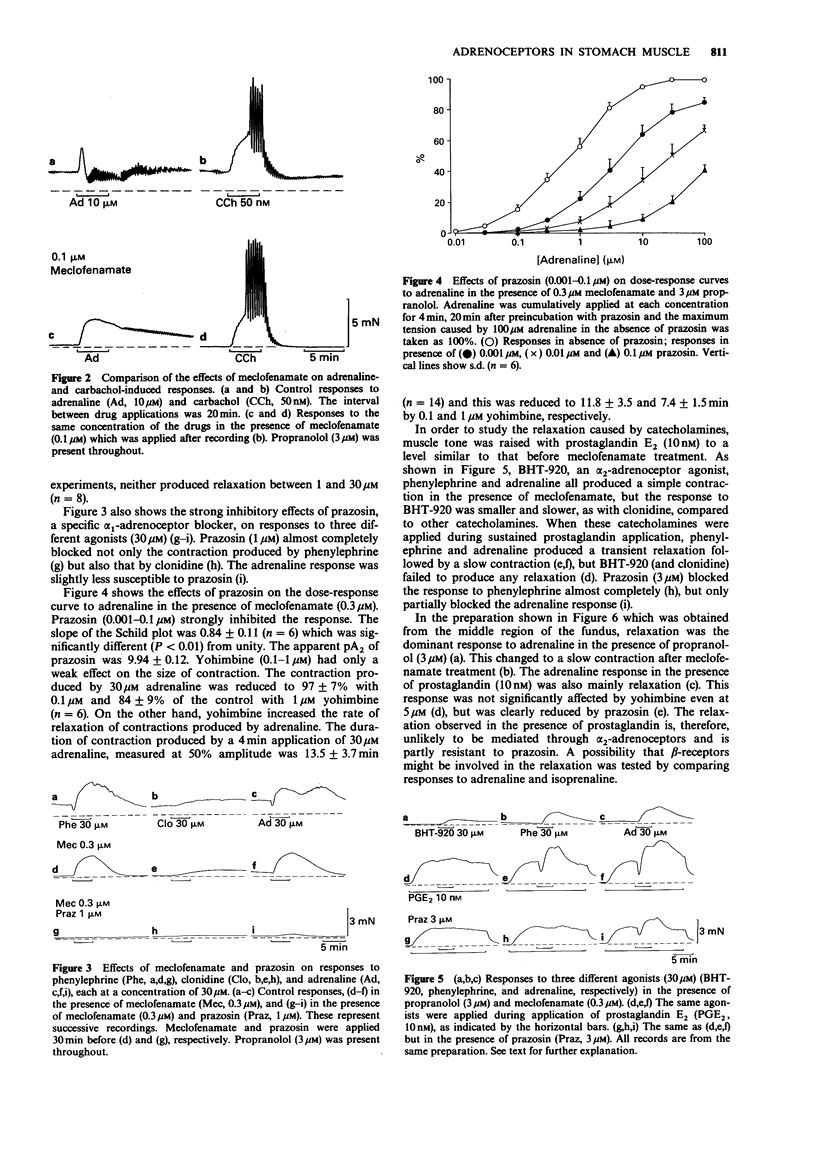
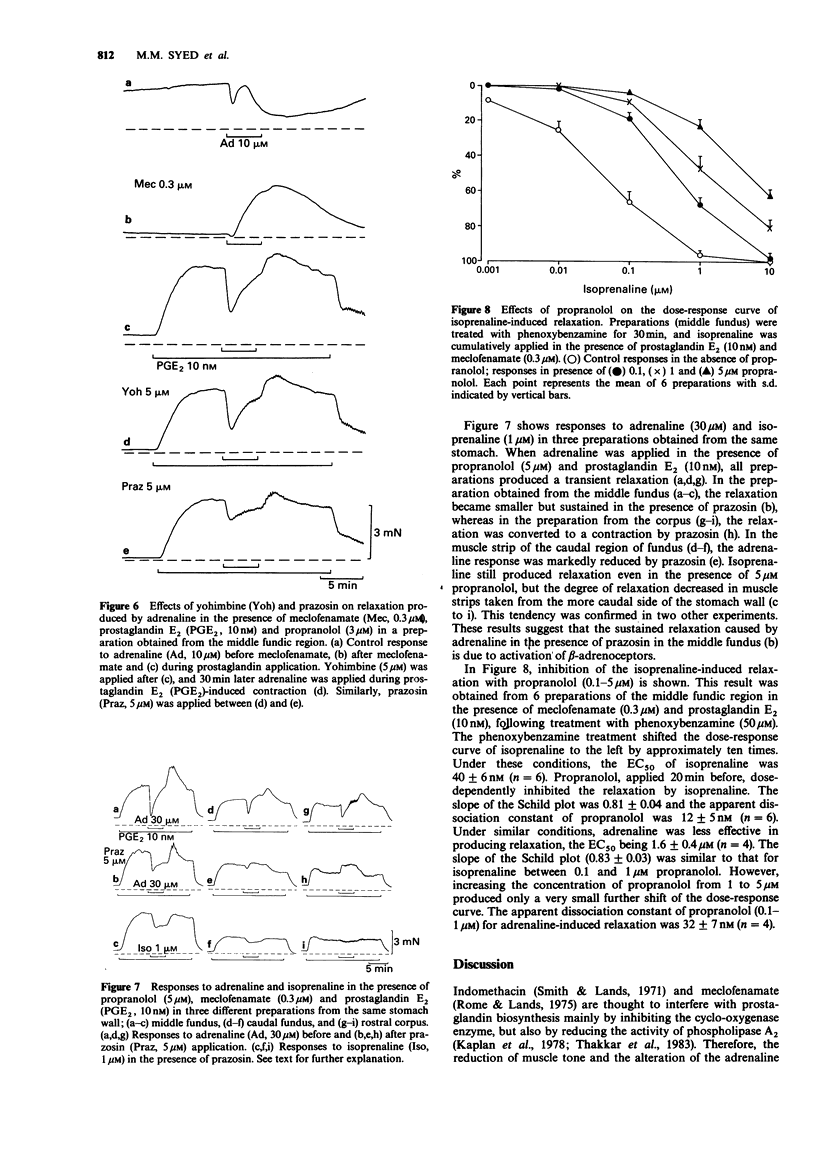
1. In circular muscle strips of the fundus and corpus of guinea-pig stomach, mechanical responses to catecholamines were studied mainly in the presence of a prostaglandin biosynthesis inhibitor, meclofenamate. 2. Normal preparations developed considerable muscle tone, and adrenaline (10-100 microM) in the presence of 3-5 microM propranolol produced a multiphasic response, generally consisting of transient relaxation and contraction, followed by slow relaxation and then contraction. Responses to phenylephrine were similar to those of adrenaline. 3. Meclofenamate (0.3 microM) nearly abolished the muscle tone and under this condition, both adrenaline and phenylephrine produced a simple contraction. This response was strongly inhibited by prazosin, but only weakly by yohimbine. 4. When muscle tone was maintained by prostaglandin E2 (10 nM) in the presence of meclofenamate, phenylephrine (30 microM) produced transient relaxation followed by slow contraction in most preparations. These were strongly inhibited by prazosin. Adrenaline produced a similar response, but the relaxation was only partially reduced by prazosin. The remaining relaxation was more dominant in the middle fundic region and this was considered to be mediated through beta-adrenoceptors. 5. It is concluded that in the circular muscle of the fundic region of guinea-pig stomach, endogenous prostaglandins are involved in maintaining muscle tone and in modifying the response to catecholamines and that both contraction and relaxation are mediated by alpha 1-adrenoceptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal D. K., Triggle C. R., Daniel E. E. Pharmacological characterization of the postsynaptic alpha adrenoceptors in vascular smooth muscle from canine and rat mesenteric vascular beds. J Pharmacol Exp Ther. 1984 Jun;229(3):831–838. [PubMed] [Google Scholar]
- Bailey D. M. Inhibitory and excitatory effects of sympathomimetic amines on muscle strips from the stomach of the guinea-pig. Br J Pharmacol. 1971 Feb;41(2):227–238. doi: 10.1111/j.1476-5381.1971.tb08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond R. A., Clarke D. E. Agonist and antagonist characterization of a putative adrenoceptor with distinct pharmacological properties from the alpha- and beta-subtypes. Br J Pharmacol. 1988 Nov;95(3):723–734. doi: 10.1111/j.1476-5381.1988.tb11698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara S., Tomita T. Mechanical and electrical responses to alpha-adrenoceptor activation in the circular muscle of guinea-pig stomach. Br J Pharmacol. 1987 Aug;91(4):789–798. doi: 10.1111/j.1476-5381.1987.tb11277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan N. A., Rimele T. J., Cooke J. P., Vanhoutte P. M. Characterization of postjunctional alpha-1 and alpha-2 adrenoceptors activated by exogenous or nerve-released norepinephrine in the canine saphenous vein. J Pharmacol Exp Ther. 1984 Sep;230(3):699–705. [PubMed] [Google Scholar]
- Frankhuijzen A. L., Bonta I. L. Role of prostaglandins in tone and effector reactivity of the isolated rat stomach preparation. Eur J Pharmacol. 1975 Mar;31(1):44–52. doi: 10.1016/0014-2999(75)90077-1. [DOI] [PubMed] [Google Scholar]
- Guimarães S. Alpha excitatory, alpha inhibitory and beta inhibitory adrenergic receptors in the guinea-pig stomach. Arch Int Pharmacodyn Ther. 1969 May;179(1):188–201. [PubMed] [Google Scholar]
- Haffner J. F. -adrenergic receptor stimulation with phenylephrine in rabbit fundus muscle exposed to carbachol. Acta Pharmacol Toxicol (Copenh) 1972;31(1):121–128. doi: 10.1111/j.1600-0773.1972.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Haffner J. F. The adrenergic receptors in isolated rabbit stomach muscle. Acta Pharmacol Toxicol (Copenh) 1971;29(4):327–338. doi: 10.1111/j.1600-0773.1971.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Ho A. K., Klein D. C. Activation of alpha 1-adrenoceptors, protein kinase C, or treatment with intracellular free Ca2+ elevating agents increases pineal phospholipase A2 activity. Evidence that protein kinase C may participate in Ca2+-dependent alpha 1-adrenergic stimulation of pineal phospholipase A2 activity. J Biol Chem. 1987 Aug 25;262(24):11764–11770. [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L., Weiss J., Elsbach P. Low concentrations of indomethacin inhibit phospholipase A2 of rabbit polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2955–2958. doi: 10.1073/pnas.75.6.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre R. A., Verplanken P. A., Bogaert M. G. Pharmacological characterization of the postjunctional beta-adrenoceptors in the rat gastric fundus. Eur J Pharmacol. 1984 Oct 30;106(1):1–9. doi: 10.1016/0014-2999(84)90671-x. [DOI] [PubMed] [Google Scholar]
- McLean J. R., Gluckman M. I. On the mechanism of the pharmacologic activity of meclofenamate sodium. Arzneimittelforschung. 1983;33(4A):627–631. [PubMed] [Google Scholar]
- Milenov K., Golenhofen K. Contractile responses of longitudinal and circular smooth muscle of the canine stomach to prostaglandins E and F2alpha. Prostaglandins Leukot Med. 1982 Mar;8(3):287–300. doi: 10.1016/0262-1746(82)90051-8. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Persson C. G., Wanstall J. C. An in vitro comparison of beta-adrenoceptor stimulants on potassium-depolarized uterine preparations from guinea-pigs. Br J Pharmacol. 1978 Feb;62(2):227–233. doi: 10.1111/j.1476-5381.1978.tb08450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Responses to the beta 2-selective agonist procaterol of vascular and atrial preparations with different functional beta-adrenoceptor populations. Br J Pharmacol. 1985 Jan;84(1):227–235. [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. The contribution of extraneuronal uptake to the trachea-blood vessel selectivity of beta-adrenoceptor stimulants in vitro in guinea-pigs. Br J Pharmacol. 1976 Jul;57(3):369–373. doi: 10.1111/j.1476-5381.1976.tb07675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy R. E., Stupecky G. L., Coulombe P. R. Further evidence for a homogeneous population of beta-1-adrenoceptors in bovine coronary artery. J Pharmacol Exp Ther. 1988 Apr;245(1):67–71. [PubMed] [Google Scholar]
- Rome L. H., Lands W. E. Structural requirements for time-dependent inhibition of prostaglandin biosynthesis by anti-inflammatory drugs. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4863–4865. doi: 10.1073/pnas.72.12.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun H. A., Costall B., Naylor R. J. Benzamide action at alpha 2-adrenoceptors modifies catecholamine-induced contraction and relaxation of circular smooth muscle from guinea-pig stomach. Naunyn Schmiedebergs Arch Pharmacol. 1982 Apr;319(1):8–11. doi: 10.1007/BF00491470. [DOI] [PubMed] [Google Scholar]
- Sahyoun H. A., Costall B., Naylor R. J. Catecholamines act as alpha 2-adrenoceptors to cause contraction of circular smooth muscle of guinea-pig stomach. J Pharm Pharmacol. 1982 Jun;34(6):381–385. doi: 10.1111/j.2042-7158.1982.tb04734.x. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Stimulation and blockade of prostaglandin biosynthesis. J Biol Chem. 1971 Nov;246(21):6700–6702. [PubMed] [Google Scholar]
- Thakkar J. K., Sperelakis N., Pang D., Franson R. C. Characterization of phospholipase A2 activity in rat aorta smooth muscle cells. Biochim Biophys Acta. 1983 Jan 7;750(1):134–140. doi: 10.1016/0005-2760(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Trachte G. J. Adrenergic receptors mediating prostaglandin production in the rabbit vas deferens. Prostaglandins. 1987 Jan;33(1):25–35. doi: 10.1016/0090-6980(87)90302-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Tomita T. Mechanical responses to catecholamines in isolated strips of the guinea-pig stomach muscle. Jpn J Pharmacol. 1974 Dec;24(6):911–922. doi: 10.1254/jjp.24.911. [DOI] [PubMed] [Google Scholar]


