Abstract
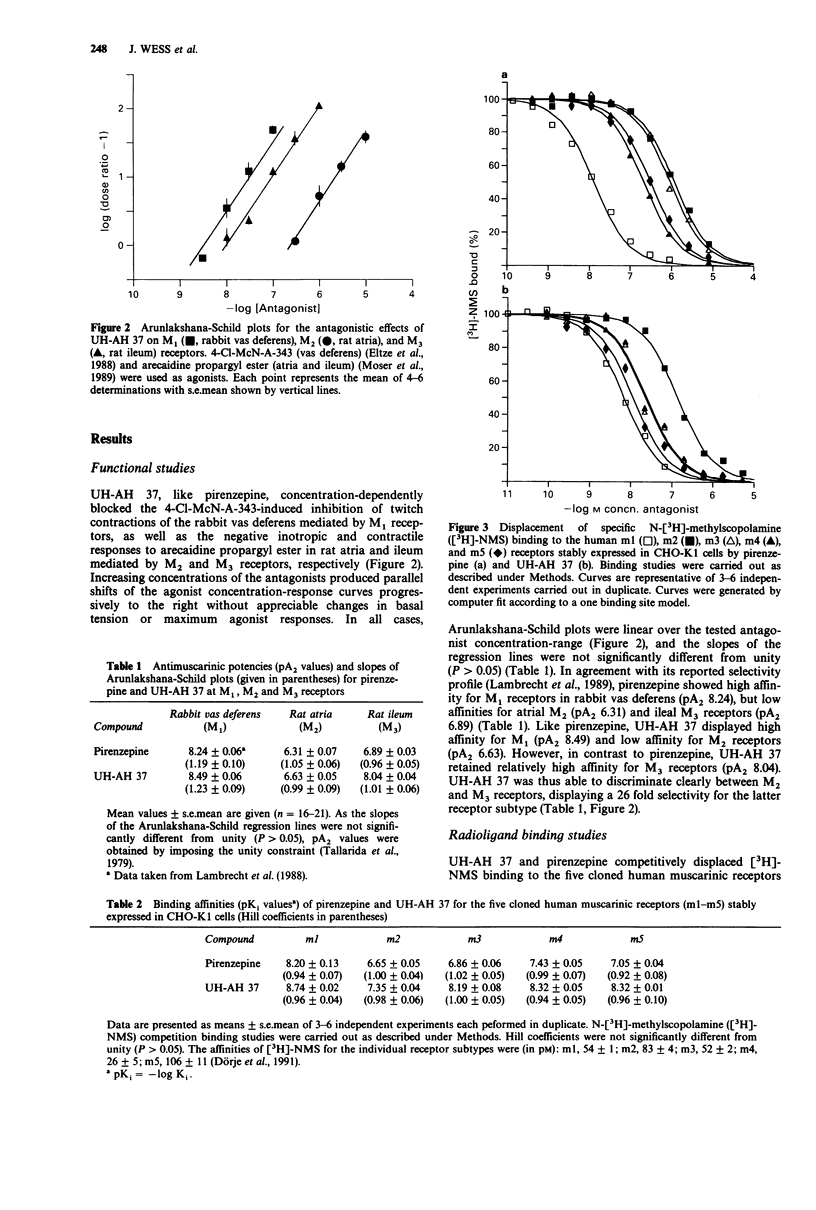
1. Functional in vitro experiments were carried out to determine the antimuscarinic potencies of the pirenzepine derivative UH-AH 37 (6-chloro-5,10-dihydro-5-[(1-methyl-4-piperidinyl)acetyl]-11H-dibenzo- [b,e] [1,4] diazepine-11-one hydrochloride) at M1 muscarinic receptors of rabbit vas deferens, M2 receptors of rat left atria and M3 receptors of rat ileum. Furthermore, N-[3H]-methylscopolamine competition binding experiments were performed to obtain its affinities for the five cloned human muscarinic receptors (m1-m5) stably expressed in CHO-K1 cells. Pirenzepine served as a reference drug throughout all experiments. 2. In all preparations used, UH-AH 37 interacted with muscarinic receptors in a fashion characteristic of a simple competitive antagonist. 3. In the functional studies, UH-AH 37, like pirenzepine, showed high affinity for M1 (pA2 8.49) and low affinity for M2 muscarinic receptors (pA2 6.63). In contrast to pirenzepine, UH-AH 37 also displayed high affinity for M3 receptors (pA2 8.04). 4. In agreement with its functional profile, UH-AH 37 bound with highest affinity to m1 (pKi 8.74) and with lowest affinity to m2 receptors (pKi 7.35). Moreover, it showed a 7 fold higher affinity for m3 (pKi 8.19) than for m2 receptors, whereas pirenzepine bound to both receptors with low affinities. 5. The binding affinity of UH-AH 37 for m4 and m5 receptors (pKi 8.32 for both receptors) was only ca. 2.5 fold lower than that for m1 receptors, while the corresponding affinity differences were 6 and 13 fold in case of pirenzepine.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba I., Kubo T., Maeda A., Bujo H., Nakai J., Mishina M., Numa S. Primary structure of porcine muscarinic acetylcholine receptor III and antagonist binding studies. FEBS Lett. 1988 Aug 1;235(1-2):257–261. doi: 10.1016/0014-5793(88)81274-2. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Shepherd M. K. A search for selective antagonists at M2 muscarinic receptors. Br J Pharmacol. 1985 Jun;85(2):427–435. doi: 10.1111/j.1476-5381.1985.tb08878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Doods H. N., Mathy M. J., Davidesko D., van Charldorp K. J., de Jonge A., van Zwieten P. A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987 Jul;242(1):257–262. [PubMed] [Google Scholar]
- Doods H. N., Mayer N. UH-AH 37, an ileal-selective muscarinic antagonist that does not discriminate between M2 and M3 binding sites. Eur J Pharmacol. 1989 Feb 28;161(2-3):215–218. doi: 10.1016/0014-2999(89)90846-7. [DOI] [PubMed] [Google Scholar]
- Eberlein W. G., Engel W., Mihm G., Rudolf K., Wetzel B., Entzeroth M., Mayer N., Doods H. N. Structure-activity relationships and pharmacological profile of selective tricyclic antimuscarinics. Trends Pharmacol Sci. 1989 Dec;Suppl:50–54. [PubMed] [Google Scholar]
- Eltz M., Gmelin G., Wess J., Strohmann C., Tacke R., Mutschler E., Lambrecht G. Presynaptic muscarinic receptors mediating inhibition of neurogenic contractions in rabbit vas deferens are of the ganglionic M1-type. Eur J Pharmacol. 1988 Dec 13;158(3):233–242. doi: 10.1016/0014-2999(88)90072-6. [DOI] [PubMed] [Google Scholar]
- Eltze M. Muscarinic M1- and M2-receptors mediating opposite effects on neuromuscular transmission in rabbit vas deferens. Eur J Pharmacol. 1988 Jul 7;151(2):205–221. doi: 10.1016/0014-2999(88)90801-1. [DOI] [PubMed] [Google Scholar]
- Giachetti A., Micheletti R., Montagna E. Cardioselective profile of AF-DX 116, a muscarine M2 receptor antagonist. Life Sci. 1986 May 5;38(18):1663–1672. doi: 10.1016/0024-3205(86)90410-8. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giraldo E., Schiavi G. B., Monferini E., Ladinsky H. Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci. 1986 May 5;38(18):1653–1662. doi: 10.1016/0024-3205(86)90409-1. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kubo T., Maeda A., Sugimoto K., Akiba I., Mikami A., Takahashi H., Haga T., Haga K., Ichiyama A., Kangawa K. Primary structure of porcine cardiac muscarinic acetylcholine receptor deduced from the cDNA sequence. FEBS Lett. 1986 Dec 15;209(2):367–372. doi: 10.1016/0014-5793(86)81144-9. [DOI] [PubMed] [Google Scholar]
- Lambrecht G., Feifel R., Forth B., Strohmann C., Tacke R., Mutschler E. p-fluoro-hexahydro-sila-difenidol: the first M2 beta-selective muscarinic antagonist. Eur J Pharmacol. 1988 Jul 26;152(1-2):193–194. doi: 10.1016/0014-2999(88)90856-4. [DOI] [PubMed] [Google Scholar]
- Lambrecht G., Feifel R., Wagner-Röder M., Strohmann C., Zilch H., Tacke R., Waelbroeck M., Christophe J., Boddeke H., Mutschler E. Affinity profiles of hexahydro-sila-difenidol analogues at muscarinic receptor subtypes. Eur J Pharmacol. 1989 Sep 1;168(1):71–80. doi: 10.1016/0014-2999(89)90634-1. [DOI] [PubMed] [Google Scholar]
- Maeda A., Kubo T., Mishina M., Numa S. Tissue distribution of mRNAs encoding muscarinic acetylcholine receptor subtypes. FEBS Lett. 1988 Nov 7;239(2):339–342. doi: 10.1016/0014-5793(88)80947-5. [DOI] [PubMed] [Google Scholar]
- Melchiorre C., Angeli P., Lambrecht G., Mutschler E., Picchio M. T., Wess J. Antimuscarinic action of methoctramine, a new cardioselective M-2 muscarinic receptor antagonist, alone and in combination with atropine and gallamine. Eur J Pharmacol. 1987 Dec 1;144(2):117–124. doi: 10.1016/0014-2999(87)90509-7. [DOI] [PubMed] [Google Scholar]
- Mitchelson F. Muscarinic receptor differentiation. Pharmacol Ther. 1988;37(3):357–423. doi: 10.1016/0163-7258(88)90005-8. [DOI] [PubMed] [Google Scholar]
- Moser U., Lambrecht G., Wagner M., Wess J., Mutschler E. Structure-activity relationships of new analogues of arecaidine propargyl ester at muscarinic M1 and M2 receptor subtypes. Br J Pharmacol. 1989 Feb;96(2):319–324. doi: 10.1111/j.1476-5381.1989.tb11820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler E., Hultzsch K. Uber Struktur-Wirkungs-Beziehungen von ungesättigten Estern des Arecaidins und Dihydroarecaidins. Arzneimittelforschung. 1973 May;23(5):732–737. [PubMed] [Google Scholar]
- Nelson W. L., Freeman D. S., Vincenzi F. F. Stereochemical analogs of a muscarinic, ganglionic stimulant. 2. Cis and trans olefinic, epoxide, and cyclopropane analogs related to 4-[N-(3-chlorophenyl)carbamoyloxy]-2-butynyltrimethylammonium chloride (McN-A-343). J Med Chem. 1976 Jan;19(1):153–158. doi: 10.1021/jm00223a026. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida R. J., Cowan A., Adler M. W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979 Aug 20;25(8):637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J. M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Waelbroeck M., Camus J., Tastenoy M., Christophe J. 80% of muscarinic receptors expressed by the NB-OK 1 human neuroblastoma cell line show high affinity for pirenzepine and are comparable to rat hippocampus M1 receptors. FEBS Lett. 1988 Jan 4;226(2):287–290. doi: 10.1016/0014-5793(88)81441-8. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Tastenoy M., Camus J., Christophe J., Strohmann C., Linoh H., Zilch H., Tacke R., Mutschler E., Lambrecht G. Binding and functional properties of antimuscarinics of the hexocyclium/sila-hexocyclium and hexahydro-diphenidol/hexahydro-sila-diphenidol type to muscarinic receptor subtypes. Br J Pharmacol. 1989 Sep;98(1):197–205. doi: 10.1111/j.1476-5381.1989.tb16882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J., Angeli P., Melchiorre C., Moser U., Mutschler E., Lambrecht G. Methoctramine selectively blocks cardiac muscarinic M2 receptors in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):246–249. doi: 10.1007/BF00173395. [DOI] [PubMed] [Google Scholar]


