Abstract
1. The effect of viral infection on the function of neuronal M2 muscarinic autoreceptors in the lungs was studied in anaesthetized guinea-pigs. 2. Guinea-pigs were inoculated intranasally with either parainfluenza type 3 or with a vehicle control. Four days later the animals were anaesthetized, paralysed and artificially ventilated. Pulmonary inflation pressure, tidal volume, blood pressure, and heart rate were recorded. Both vagus nerves were cut and electrical stimulation of the distal portions caused bronchoconstriction (measured as an increase in pulmonary inflation pressure) and bradycardia. 3. In control animals, pilocarpine (1-100 micrograms kg-1, i.v.) attenuated vagally-induced bronchoconstriction by stimulating inhibitory M2 muscarinic receptors on parasympathetic nerves in the lungs. Conversely, blockade of these receptors with the antagonist gallamine (0.1-10 mg kg-1, i.v.) produced a marked potentiation of vagally-induced bronchoconstriction. These results confirm previous findings. 4. In guinea-pigs infected with parainfluenza virus, pilocarpine did not inhibit vagally-induced bronchoconstriction. Furthermore, gallamine did not potentiate vagally-induced bronchoconstriction to the same degree as in uninfected controls. 5. There was no increase in baseline pulmonary inflation pressure in the infected animals over the controls. Receptors on airway smooth muscle were unchanged by viral infection since large doses of pilocarpine caused equivalent bronchoconstriction in both groups of animals. Gallamine inhibited the vagally-induced fall in heart rate equally in both groups of animals indicating that virus-induced changes in M2 receptor function on pulmonary parasympathetic nerves are not part of a generalized decrease in M2 receptor function.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

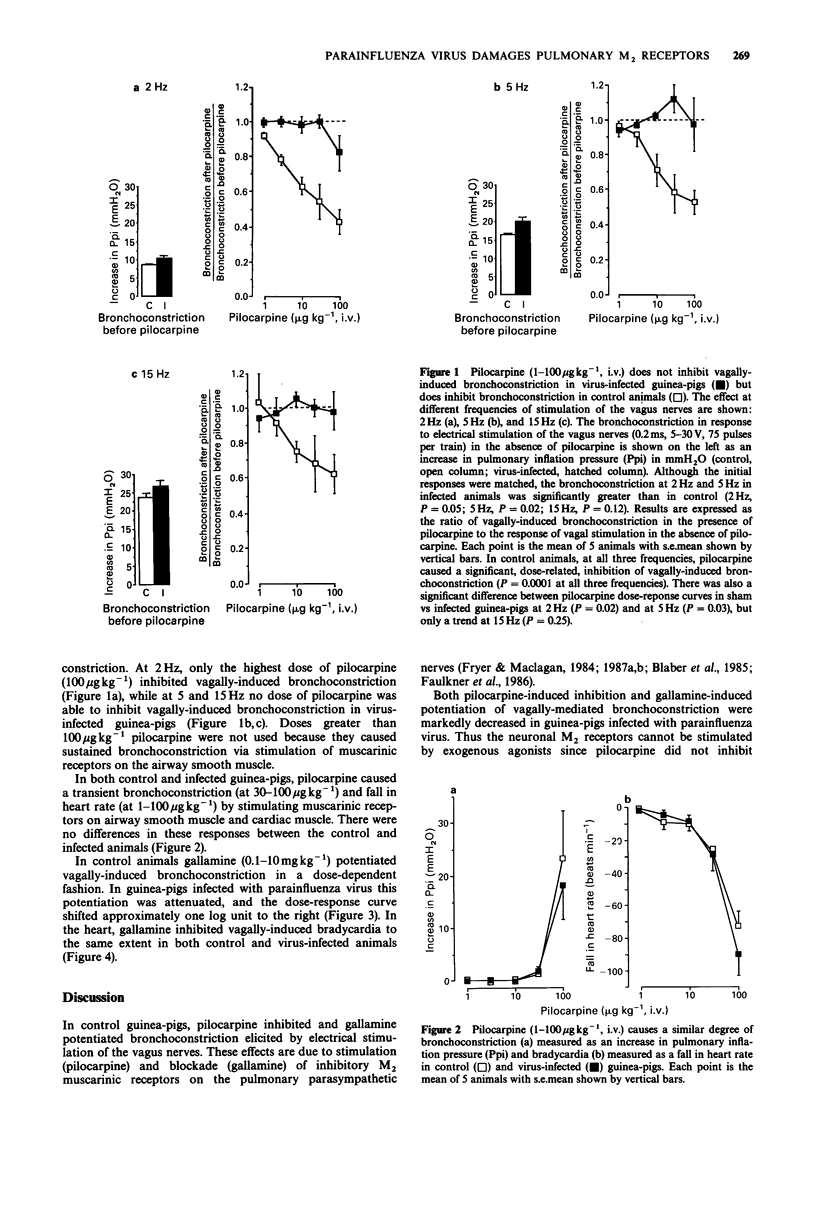
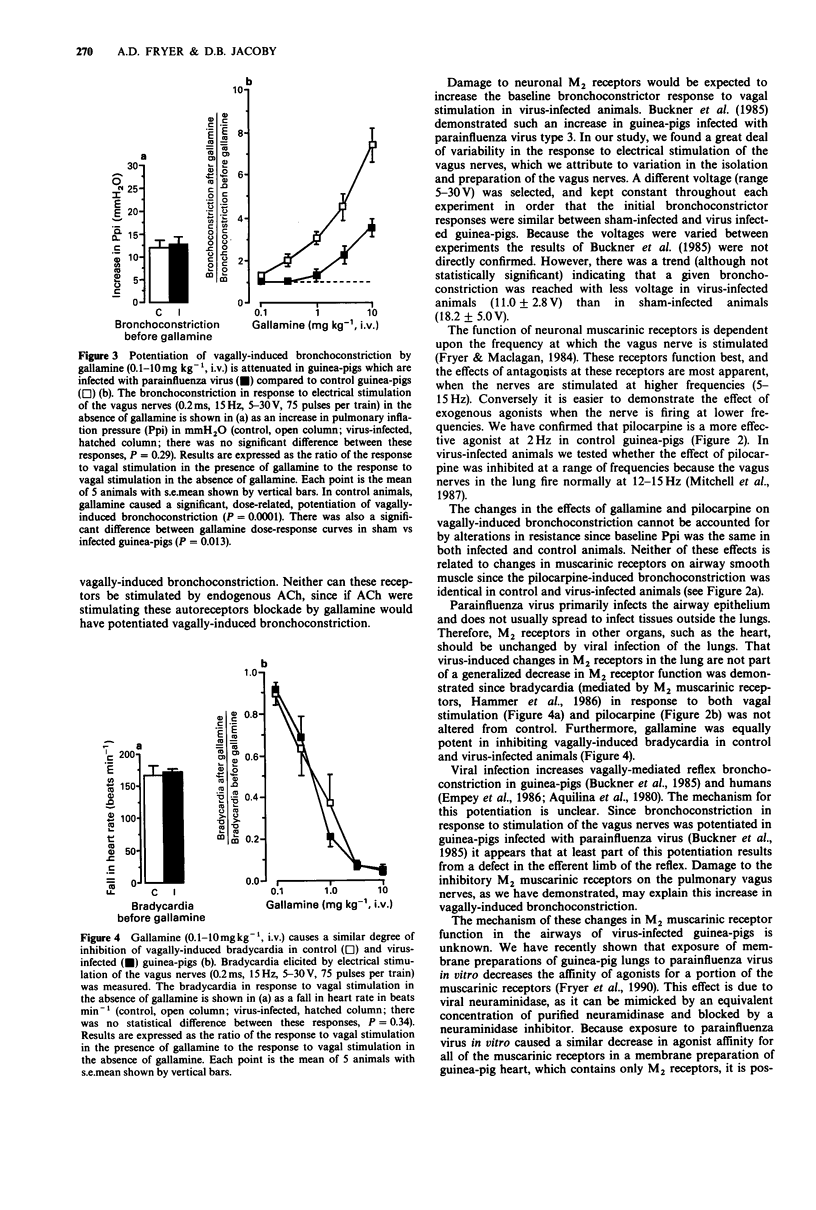

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquilina A. T., Hall W. J., Douglas R. G., Jr, Utell M. J. Airway reactivity in subjects with viral upper respiratory tract infections: the effects of exercise and cold air. Am Rev Respir Dis. 1980 Jul;122(1):3–10. doi: 10.1164/arrd.1980.122.1.3. [DOI] [PubMed] [Google Scholar]
- Blaber L. C., Fryer A. D. The response of cat airways to histamine in vivo and in vitro. Br J Pharmacol. 1985 Feb;84(2):309–316. doi: 10.1111/j.1476-5381.1985.tb12915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. T., Greenberg S. B., Stevens P. M., Bilunos P. A., Couch R. B. Effects of rhinovirus infection of pulmonary function of healthy human volunteers. Am Rev Respir Dis. 1976 Jul;114(1):95–102. doi: 10.1164/arrd.1976.114.1.95. [DOI] [PubMed] [Google Scholar]
- Buckner C. K., Clayton D. E., Ain-Shoka A. A., Busse W. W., Dick E. C., Shult P. Parainfluenza 3 infection blocks the ability of a beta adrenergic receptor agonist to inhibit antigen-induced contraction of guinea pig isolated airway smooth muscle. J Clin Invest. 1981 Feb;67(2):376–384. doi: 10.1172/JCI110045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner C. K., Songsiridej V., Dick E. C., Busse W. W. In vivo and in vitro studies on the use of the guinea pig as a model for virus-provoked airway hyperreactivity. Am Rev Respir Dis. 1985 Aug;132(2):305–310. doi: 10.1164/arrd.1985.132.2.305. [DOI] [PubMed] [Google Scholar]
- Burden D. T., Parkes M. W., Gardiner D. G. Effect of beta-adrenoceptive blocking agents on the response to bronchoconstrictor drugs in the guinea-pig air overflow preparation. Appendix describing a new modification of the air overflow method. Br J Pharmacol. 1971 Jan;41(1):122–131. doi: 10.1111/j.1476-5381.1971.tb09942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W. E. Contributions to the physiology of the lungs: Part I. The bronchial muscles, their innervation, and the action of drugs upon them. J Physiol. 1903 Mar 16;29(2):97–173. doi: 10.1113/jphysiol.1903.sp000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusser D. J., Jacoby D. B., Djokic T. D., Rubinstein I., Borson D. B., Nadel J. A. Virus induces airway hyperresponsiveness to tachykinins: role of neutral endopeptidase. J Appl Physiol (1985) 1989 Oct;67(4):1504–1511. doi: 10.1152/jappl.1989.67.4.1504. [DOI] [PubMed] [Google Scholar]
- Empey D. W., Laitinen L. A., Jacobs L., Gold W. M., Nadel J. A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976 Feb;113(2):131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- Faulkner D., Fryer A. D., Maclagan J. Postganglionic muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1986 May;88(1):181–187. doi: 10.1111/j.1476-5381.1986.tb09485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick O. L., German D. F., Mills J. Development of allergy in children. I. Association with virus infections. J Allergy Clin Immunol. 1979 Apr;63(4):228–241. doi: 10.1016/0091-6749(79)90106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A. D., Maclagan J. Ipratropium bromide potentiates bronchoconstriction induced by vagal nerve stimulation in the guinea-pig. Eur J Pharmacol. 1987 Jul 9;139(2):187–191. doi: 10.1016/0014-2999(87)90251-2. [DOI] [PubMed] [Google Scholar]
- Fryer A. D., Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984 Dec;83(4):973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A. D., Maclagan J. Pancuronium and gallamine are antagonists for pre- and post-junctional muscarinic receptors in the guinea-pig lung. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):367–371. doi: 10.1007/BF00165549. [DOI] [PubMed] [Google Scholar]
- Fryer A. D., el-Fakahany E. E., Jacoby D. B. Parainfluenza virus type 1 reduces the affinity of agonists for muscarinic receptors in guinea-pig lung and heart. Eur J Pharmacol. 1990 May 31;181(1-2):51–58. doi: 10.1016/0014-2999(90)90244-z. [DOI] [PubMed] [Google Scholar]
- Gies J. P., Landry Y. Sialic acid is selectively involved in the interaction of agonists with M2 muscarinic acetylcholine receptors. Biochem Biophys Res Commun. 1988 Jan 29;150(2):673–680. doi: 10.1016/0006-291x(88)90444-5. [DOI] [PubMed] [Google Scholar]
- Hall W. J., Douglas R. G., Jr, Hyde R. W., Roth F. K., Cross A. S., Speers D. M. Pulmonary mechanics after uncomplicated influenza A infection. Am Rev Respir Dis. 1976 Feb;113(2):141–148. doi: 10.1164/arrd.1976.113.2.141. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giraldo E., Schiavi G. B., Monferini E., Ladinsky H. Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci. 1986 May 5;38(18):1653–1662. doi: 10.1016/0024-3205(86)90409-1. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Clyde W. A., Jr, Collier A. M., Denny F. W., Senior R. J., Sheaffer C. I., Conley W. G., 3rd, Christian R. M. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. J Pediatr. 1979 Aug;95(2):183–190. doi: 10.1016/s0022-3476(79)80647-2. [DOI] [PubMed] [Google Scholar]
- Jacoby D. B., Tamaoki J., Borson D. B., Nadel J. A. Influenza infection causes airway hyperresponsiveness by decreasing enkephalinase. J Appl Physiol (1985) 1988 Jun;64(6):2653–2658. doi: 10.1152/jappl.1988.64.6.2653. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Pierce A. K., Sanford J. P. Pulmonary function in uncomplicated influenza. Am Rev Respir Dis. 1969 Aug;100(2):141–146. doi: 10.1164/arrd.1969.100.2.141. [DOI] [PubMed] [Google Scholar]
- Little J. W., Hall W. J., Douglas R. G., Jr, Mudholkar G. S., Speers D. M., Patel K. Airway hyperreactivity and peripheral airway dysfunction in influenza A infection. Am Rev Respir Dis. 1978 Aug;118(2):295–303. doi: 10.1164/arrd.1978.118.2.295. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A., Baker D. G., Basbaum C. B. In vivo activity of tracheal parasympathetic ganglion cells innervating tracheal smooth muscle. Brain Res. 1987 Dec 22;437(1):157–160. doi: 10.1016/0006-8993(87)91537-x. [DOI] [PubMed] [Google Scholar]
- Peterson G. L., Rosenbaum L. C., Broderick D. J., Schimerlik M. I. Physical properties of the purified cardiac muscarinic acetylcholine receptor. Biochemistry. 1986 Jun 3;25(11):3189–3202. doi: 10.1021/bi00359a017. [DOI] [PubMed] [Google Scholar]
- Picken J. J., Niewoehner D. E., Chester E. H. Prolonged effects of viral infections of the upper respiratory tract upon small airways. Am J Med. 1972 Jun;52(6):738–746. doi: 10.1016/0002-9343(72)90079-4. [DOI] [PubMed] [Google Scholar]
- SHELOKOV A., VOGEL J. E., CHI L. Hemadsorption (adsorption-hemagglutination) test for viral agents in tissue culture with special reference to influenza. Proc Soc Exp Biol Med. 1958 Apr;97(4):802–809. doi: 10.3181/00379727-97-23884. [DOI] [PubMed] [Google Scholar]
- Welliver R. C. Upper respiratory infections in asthma. J Allergy Clin Immunol. 1983 Oct;72(4):341–346. doi: 10.1016/0091-6749(83)90497-9. [DOI] [PubMed] [Google Scholar]



