Abstract
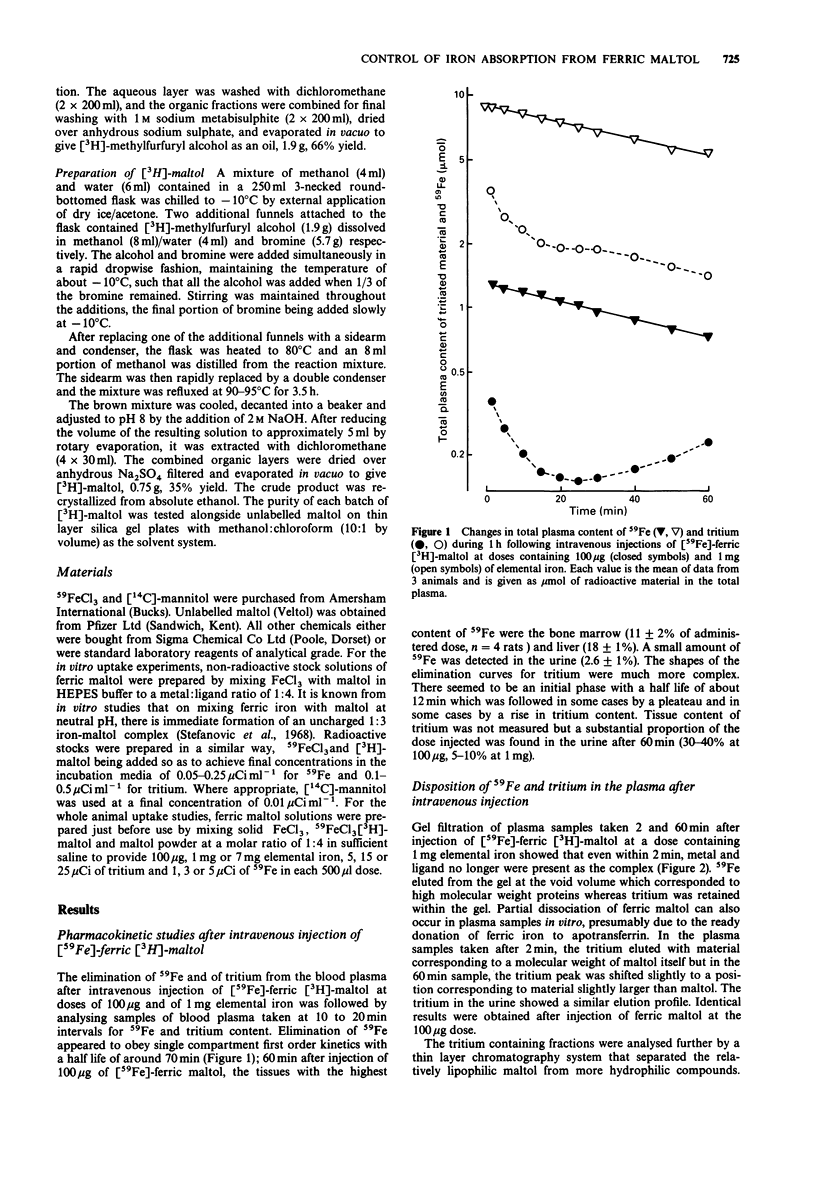
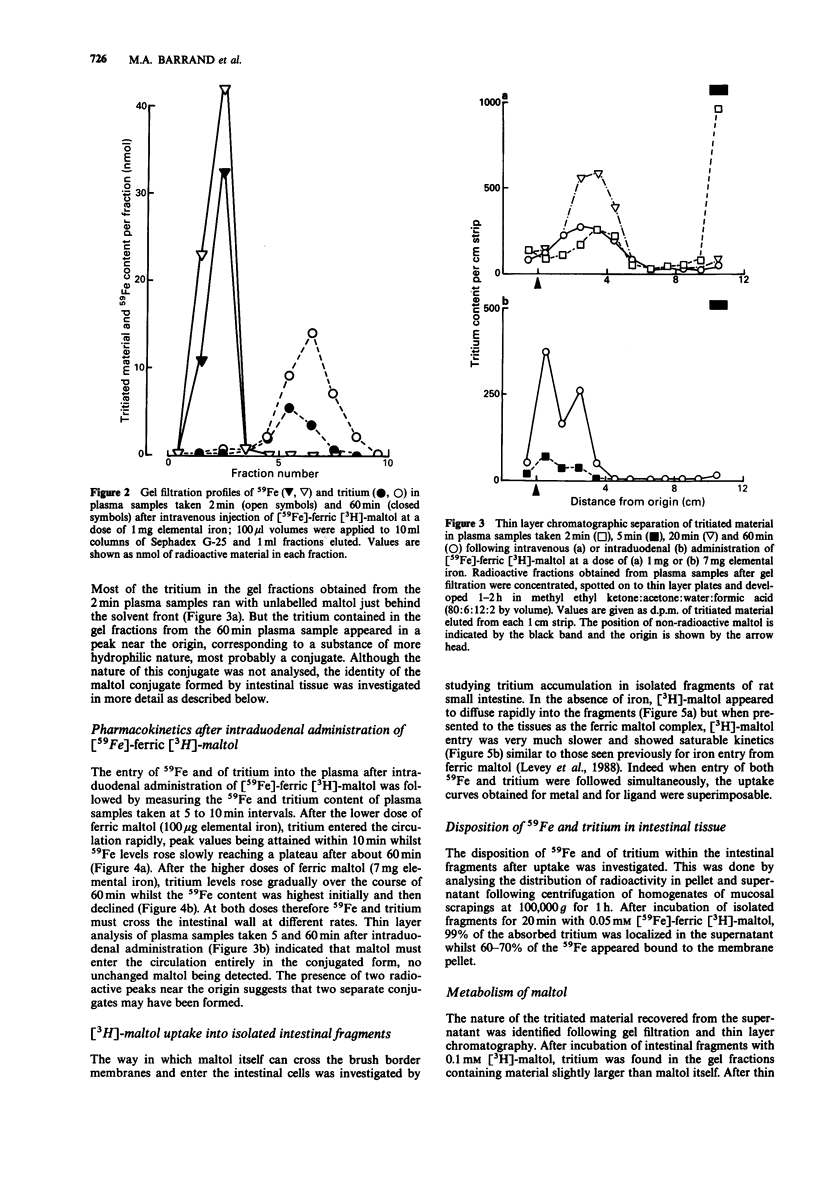
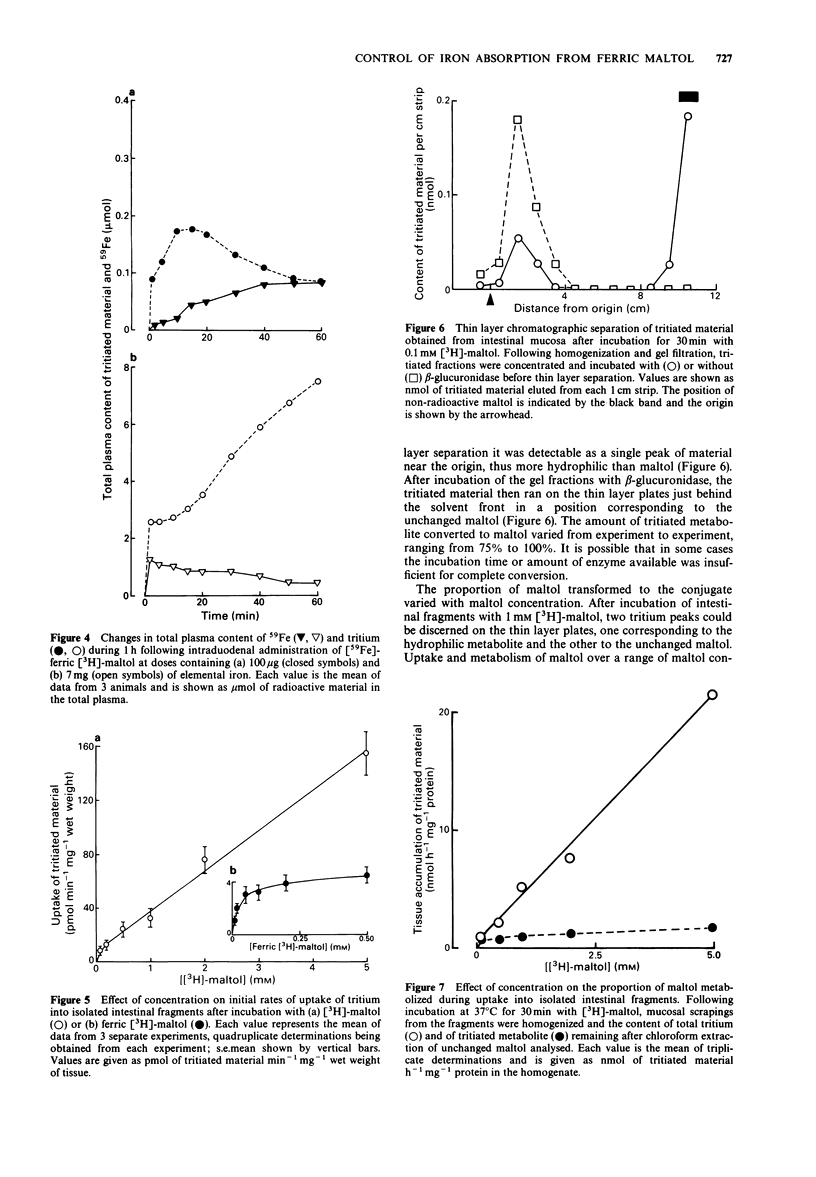
1. The fate and disposition of [59Fe]-ferric [3H]-maltol after intravenous administration were investigated in anaesthetized rats. Immediate dissociation of ferric iron from maltol took place in the circulation even with high doses of ferric maltol (containing 1 mg elemental iron). In plasma samples withdrawn within 1 min of injection and subjected to gel filtration, 59Fe eluted with the high molecular weight proteins whilst the tritium was associated with low molecular weight material. 2. The rates of elimination of 59Fe and of tritium from the plasma and their ultimate fate were very different. The half life for 59Fe in the plasma was around 70 min and 59Fe appeared mainly in the bone marrow and liver. There was an initial rapid exit of tritium from the plasma with a half life of around 12 min. This was followed either by a plateau or by a rise in tritium levels, involving entry of maltol metabolites into the circulation. These metabolites could be recovered in the urine. 3. Entry of 59Fe and of tritium into the blood plasma after intraduodenal administration of [59Fe]-ferric [3H]-maltol was also very different. At low doses of ferric maltol (containing 100 micrograms elemental iron), the tritium appeared in the plasma in highest amounts within seconds and then decreased whilst there was a slow rise in 59Fe levels. At higher doses of ferric maltol (containing 7 mg elemental iron), levels of 59Fe in the plasma were highest at 5 min and then fell whereas tritium levels rose steadily. Mucosal processing of 59Fe prevented further entry of iron at high dose into the circulation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrand M. A., Callingham B. A., Hider R. C. Effects of the pyrones, maltol and ethyl maltol, on iron absorption from the rat small intestine. J Pharm Pharmacol. 1987 Mar;39(3):203–211. doi: 10.1111/j.2042-7158.1987.tb06249.x. [DOI] [PubMed] [Google Scholar]
- Barrand M. A., Hider R. C., Callingham B. A. The importance of reductive mechanisms for intestinal uptake of iron from ferric maltol and ferric nitrilotriacetic acid (NTA). J Pharm Pharmacol. 1990 Apr;42(4):279–282. doi: 10.1111/j.2042-7158.1990.tb05408.x. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Burchell B., Dutton G. J., Hänninen O., Mulder G. J., Owens I. S., Siest G., Tephly T. R. UDP-glucuronosyltransferase activities. Guidelines for consistent interim terminology and assay conditions. Biochem Pharmacol. 1983 Mar 15;32(6):953–955. doi: 10.1016/0006-2952(83)90610-x. [DOI] [PubMed] [Google Scholar]
- Brissot P., Wright T. L., Ma W. L., Weisiger R. A. Efficient clearance of non-transferrin-bound iron by rat liver. Implications for hepatic iron loading in iron overload states. J Clin Invest. 1985 Oct;76(4):1463–1470. doi: 10.1172/JCI112125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld M., Bell J. D., Halliwell B., Aruoma O. I., Bomford A., Sadler P. J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Mar 15;264(8):4417–4422. [PubMed] [Google Scholar]
- Heinrich H. C. Intestinal absorption of 59Fe from neutron-activated commercial oral iron(III)-citrate and iron(III)-hydroxide-polymaltose complexes in man. Arzneimittelforschung. 1987 Jan;37(1A):105–107. [PubMed] [Google Scholar]
- Hungerford D. M., Jr, Linder M. C. Interactions of pH and ascorbate in intestinal iron absorption. J Nutr. 1983 Dec;113(12):2615–2622. doi: 10.1093/jn/113.12.2615. [DOI] [PubMed] [Google Scholar]
- Koster A. S., Noordhoek J. Glucuronidation in the rat intestinal wall. Comparison of isolated mucosal cells, latent microsomes and activated microsomes. Biochem Pharmacol. 1983 Mar 1;32(5):895–900. doi: 10.1016/0006-2952(83)90594-4. [DOI] [PubMed] [Google Scholar]
- Levey J. A., Barrand M. A., Callingham B. A., Hider R. C. Characteristics of iron(III) uptake by isolated fragments of rat small intestine in the presence of the hydroxypyrones, maltol and ethyl maltol. Biochem Pharmacol. 1988 May 15;37(10):2051–2057. doi: 10.1016/0006-2952(88)90556-4. [DOI] [PubMed] [Google Scholar]
- Nayfield S. G., Kent T. H., Rodman N. F. Gastrointestinal effects of acute ferrous sulfate poisoning in rats. Arch Pathol Lab Med. 1976 Jun;100(6):325–328. [PubMed] [Google Scholar]
- Peters T. J., Raja K. B., Simpson R. J., Snape S. Mechanisms and regulation of intestinal iron absorption. Ann N Y Acad Sci. 1988;526:141–147. doi: 10.1111/j.1749-6632.1988.tb55500.x. [DOI] [PubMed] [Google Scholar]
- Rennhard H. H. The metabolism of ethyl maltol and maltol in the dog. J Agric Food Chem. 1971 Jan-Feb;19(1):152–154. doi: 10.1021/jf60173a036. [DOI] [PubMed] [Google Scholar]
- Singh R. K., Barrand M. A. Lipid peroxidation effects of a novel iron compound, ferric maltol. A comparison with ferrous sulphate. J Pharm Pharmacol. 1990 Apr;42(4):276–279. doi: 10.1111/j.2042-7158.1990.tb05407.x. [DOI] [PubMed] [Google Scholar]
- Slivka A., Kang J., Cohen G. Hydroxyl radicals and the toxicity of oral iron. Biochem Pharmacol. 1986 Feb 15;35(4):553–556. doi: 10.1016/0006-2952(86)90346-1. [DOI] [PubMed] [Google Scholar]
- Wright T. L., Fitz J. G., Weisiger R. A. Non-transferrin-bound iron uptake by rat liver. Role of membrane potential difference. J Biol Chem. 1988 Feb 5;263(4):1842–1847. [PubMed] [Google Scholar]


