Abstract
1. Whether the function of the postsynaptic acetylcholine receptor is use-dependently affected by repetitive nerve stimulation in the presence of competitive antagonists was studied in the mouse phrenic nerve-hemidiaphragm preparation. 2. For electrophysiological experiments, the preparation was immobilized by synthetic mu-conotoxin, which preferentially blocks muscular Na-channels causing neither depolarization of the membrane potential, inhibition of quantal transmitter release, nor depression of nicotinic receptor function. 3. High concentrations of cobratoxin depressed indirect twitches and endplate potentials (e.p.ps) without inducing waning of contractilities or run-down of trains of e.p.ps evoked at 10-100 Hz. However, waning and run-down were accelerated after washout of the toxin despite diminished postsynaptic receptor blockade. Once the run-down of e.p.ps was produced by washout or low concentrations of cobratoxin, further depression of e.p.p. amplitude with high concentrations of cobratoxin did not attenuate the e.p.p. run-down. 4. The degrees of waning of tetanus and trains of e.p.ps produced by a very high concentration of tubocurarine (20 microM) were also less than that caused at a 100 fold lower concentration, albeit the amplitudes of twitches and the first e.p.p. were depressed more rapidly and markedly. 5. Tubocurarine, like cobratoxin, depressed the amplitude of miniature endplate potentials (m.e.p.ps) more than e.p.ps. 6. In contrast to the steepened run-down of successive e.p.ps in the presence of low concentrations of either nicotinic antagonists, the amplitude of m.e.p.ps observed during repetitive stimulation was uniform and was not different from that before stimulation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
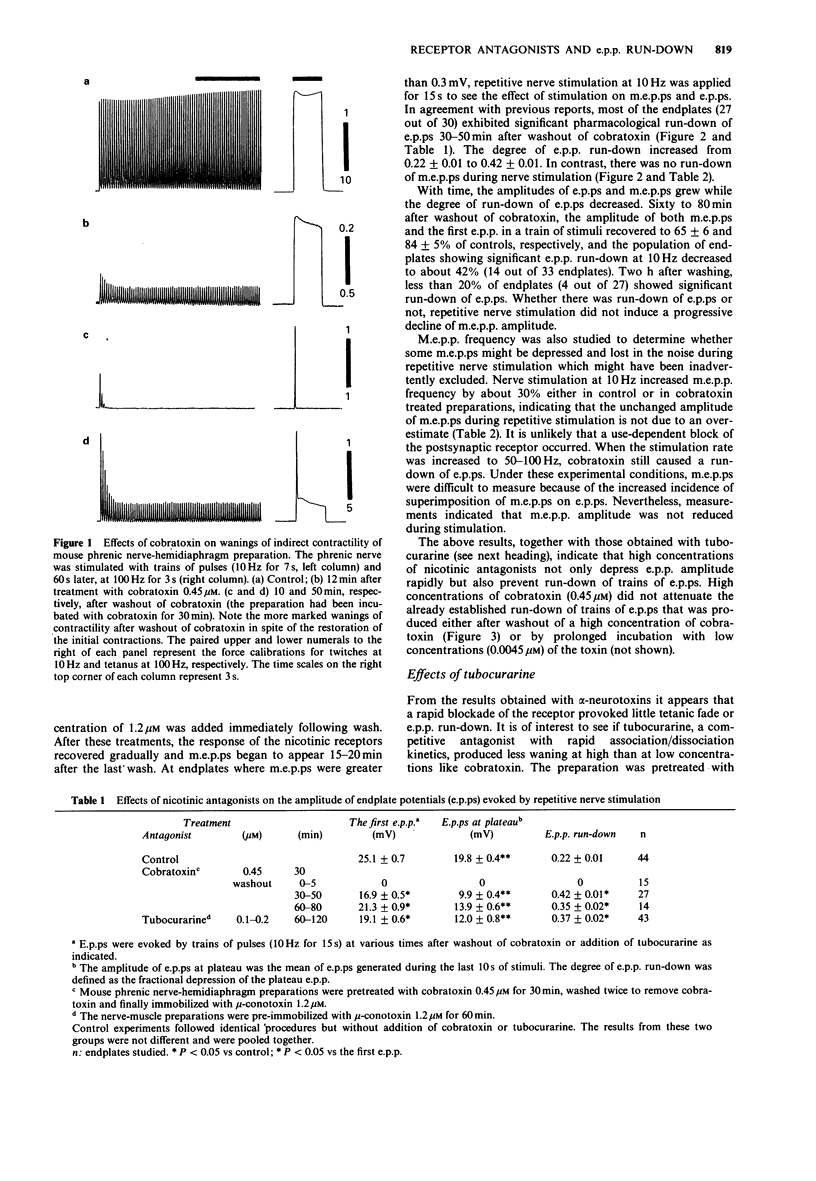
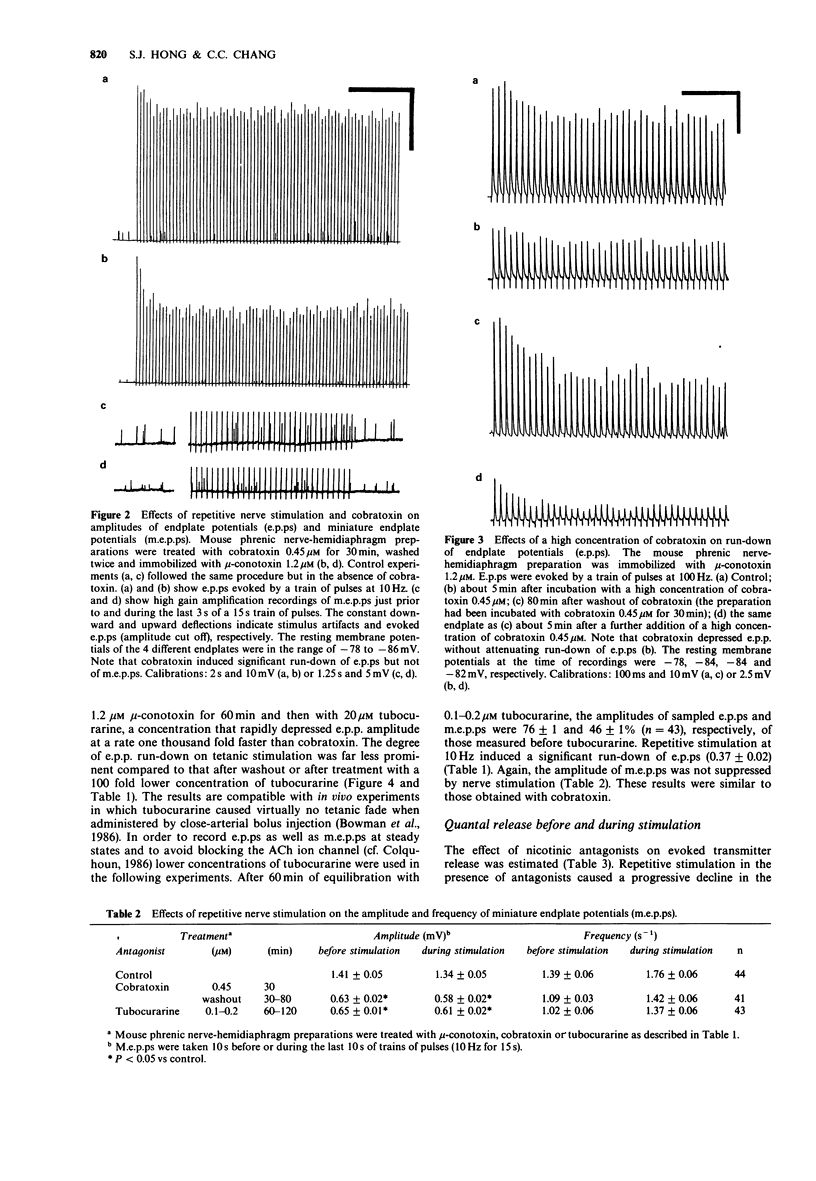
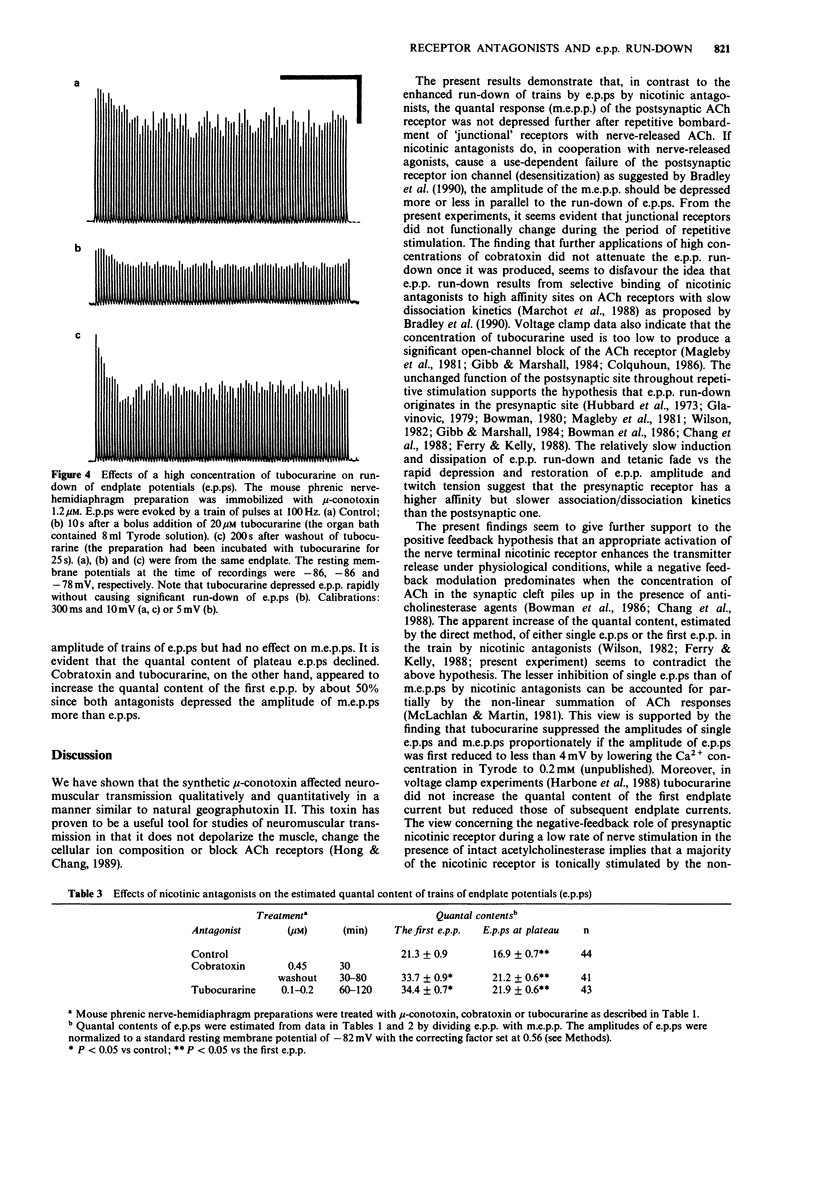
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barstad J. A., Lilleheil G. Transversaly cut diaphragm preparation from rat. An adjuvant tool in the study of the physiology and pbarmacology of the myoneural junction. Arch Int Pharmacodyn Ther. 1968 Oct;175(2):373–390. [PubMed] [Google Scholar]
- Blaber L. C. The prejunctional actions of some non-depolarizing blocking drugs. Br J Pharmacol. 1973 Jan;47(1):109–116. doi: 10.1111/j.1476-5381.1973.tb08163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C. Prejunctional and postjunctional cholinoceptors at the neuromuscular junction. Anesth Analg. 1980 Dec;59(12):935–943. [PubMed] [Google Scholar]
- Bowman W. C., Webb S. N. Tetanic fade during partial transmission failure produced by non-depolarizing neuromuscular blocking drugs in the cat. Clin Exp Pharmacol Physiol. 1976 Nov-Dec;3(6):545–555. doi: 10.1111/j.1440-1681.1976.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Bradley R. J., Edge M. T., Chau W. C. The alpha-neurotoxin erabutoxin b causes fade at the rat end-plate. Eur J Pharmacol. 1990 Jan 25;176(1):11–21. doi: 10.1016/0014-2999(90)90127-r. [DOI] [PubMed] [Google Scholar]
- Bradley R. J., Pagala M. K., Edge M. T. Multiple effects of alpha-toxins on the nicotinic acetylcholine receptor. FEBS Lett. 1987 Nov 30;224(2):277–282. doi: 10.1016/0014-5793(87)80469-6. [DOI] [PubMed] [Google Scholar]
- CHANG C. C., LEE C. Y. ISOLATION OF NEUROTOXINS FROM THE VENOM OF BUNGARUS MULTICINCTUS AND THEIR MODES OF NEUROMUSCULAR BLOCKING ACTION. Arch Int Pharmacodyn Ther. 1963 Jul 1;144:241–257. [PubMed] [Google Scholar]
- Chang C. C., Chen S. M., Hong S. J. Reversals of the neostigmine-induced tetanic fade and endplate potential run-down with respect to the autoregulation of transmitter release. Br J Pharmacol. 1988 Dec;95(4):1255–1261. doi: 10.1111/j.1476-5381.1988.tb11762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Hong S. J. Dissociation of the end-plate potential run-down and the tetanic fade from the postsynaptic inhibition of acetylcholine receptor by alpha-neurotoxins. Exp Neurol. 1987 Dec;98(3):509–517. doi: 10.1016/0014-4886(87)90260-3. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Hong S. J., Ko J. L. Mechanisms of the inhibition by neostigmine of tetanic contraction in the mouse diaphragm. Br J Pharmacol. 1986 Apr;87(4):757–762. doi: 10.1111/j.1476-5381.1986.tb14594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L. J., Gray W. R., Olivera B. M., Zeikus R. D., Kerr L., Yoshikami D., Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985 Aug 5;260(16):9280–9288. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry C. B., Kelly S. S. The nature of the presynaptic effects of (+)-tubocurarine at the mouse neuromuscular junction. J Physiol. 1988 Sep;403:425–437. doi: 10.1113/jphysiol.1988.sp017257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb A. J., Marshall I. G. Nicotinic antagonists produce differing amounts of tetanic fade in the isolated diaphragm of the rat. Br J Pharmacol. 1986 Nov;89(3):619–624. doi: 10.1111/j.1476-5381.1986.tb11164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb A. J., Marshall I. G. Pre-and post-junctional effects of tubocurarine and other nicotinic antagonists during repetitive stimulation in the rat. J Physiol. 1984 Jun;351:275–297. doi: 10.1113/jphysiol.1984.sp015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavinović M. I. Presynaptic action of curare. J Physiol. 1979 May;290(2):499–506. doi: 10.1113/jphysiol.1979.sp012786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne A. J., Bowman W. C., Marshall I. G. Effects of tubocurarine on end-plate current rundown and quantal content during rapid nerve stimulation in the snake. Clin Exp Pharmacol Physiol. 1988 Jun;15(6):479–490. doi: 10.1111/j.1440-1681.1988.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. Br J Pharmacol. 1989 Jul;97(3):934–940. doi: 10.1111/j.1476-5381.1989.tb12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S., Terrar D. A. The effect of (+)-tubocurarine on neuromuscular transmission during repetitive stimulation in the rat, mouse, and frog. J Physiol. 1981 Mar;312:97–113. doi: 10.1113/jphysiol.1981.sp013618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchot P., Frachon P., Bougis P. E. Selective distinction at equilibrium between the two alpha-neurotoxin binding sites of Torpedo acetylcholine receptor by microtitration. Eur J Biochem. 1988 Jun 15;174(3):537–542. doi: 10.1111/j.1432-1033.1988.tb14132.x. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. The methonium. Pharmacol Rev. 1952 Sep;4(3):219–253. [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Relation between subsynaptic receptor blockade and response to quantal transmitter at the mouse neuromuscular junction. J Gen Physiol. 1981 Sep;78(3):313–344. doi: 10.1085/jgp.78.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai N., Hughes R., Payne J. P. Sequential changes in the fade of tetanic tension after the administration of tubocurarine in anaesthetized man. Br J Anaesth. 1976 Jun;48(6):535–539. doi: 10.1093/bja/48.6.535. [DOI] [PubMed] [Google Scholar]
- Takagi H., Kojima M., Nagata M., Kuromi H. On the site of action of hemicholinium-3 at the rat phrenic nerve-diaphragm preparation with special reference to its multiple presynaptic actions. Neuropharmacology. 1970 Jul;9(4):359–367. doi: 10.1016/0028-3908(70)90033-x. [DOI] [PubMed] [Google Scholar]
- Wessler I., Rasbach J., Scheuer B., Hillen U., Kilbinger H. Effects of (+)-tubocurarine on [3H]acetylcholine release from the rat phrenic nerve at different stimulation frequencies and train lengths. Naunyn Schmiedebergs Arch Pharmacol. 1987 May;335(5):496–501. doi: 10.1007/BF00169114. [DOI] [PubMed] [Google Scholar]
- Wilson D. F. Influence of presynaptic receptors on neuromuscular transmission in rat. Am J Physiol. 1982 May;242(5):C366–C372. doi: 10.1152/ajpcell.1982.242.5.C366. [DOI] [PubMed] [Google Scholar]
- Zemková H., Vyskocil F., Edwards C. The effects of nerve terminal activity on non-quantal release of acetylcholine at the mouse neuromuscular junction. J Physiol. 1990 Apr;423:631–640. doi: 10.1113/jphysiol.1990.sp018044. [DOI] [PMC free article] [PubMed] [Google Scholar]


