Abstract
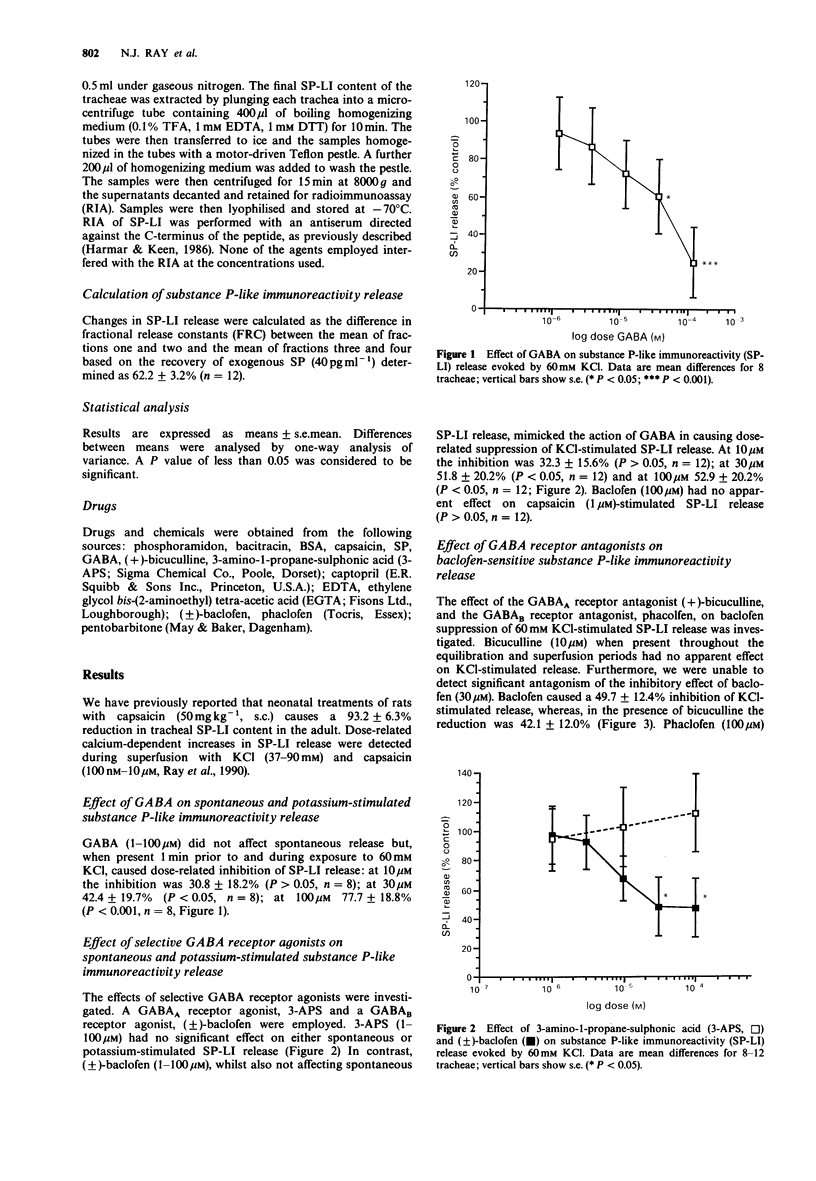
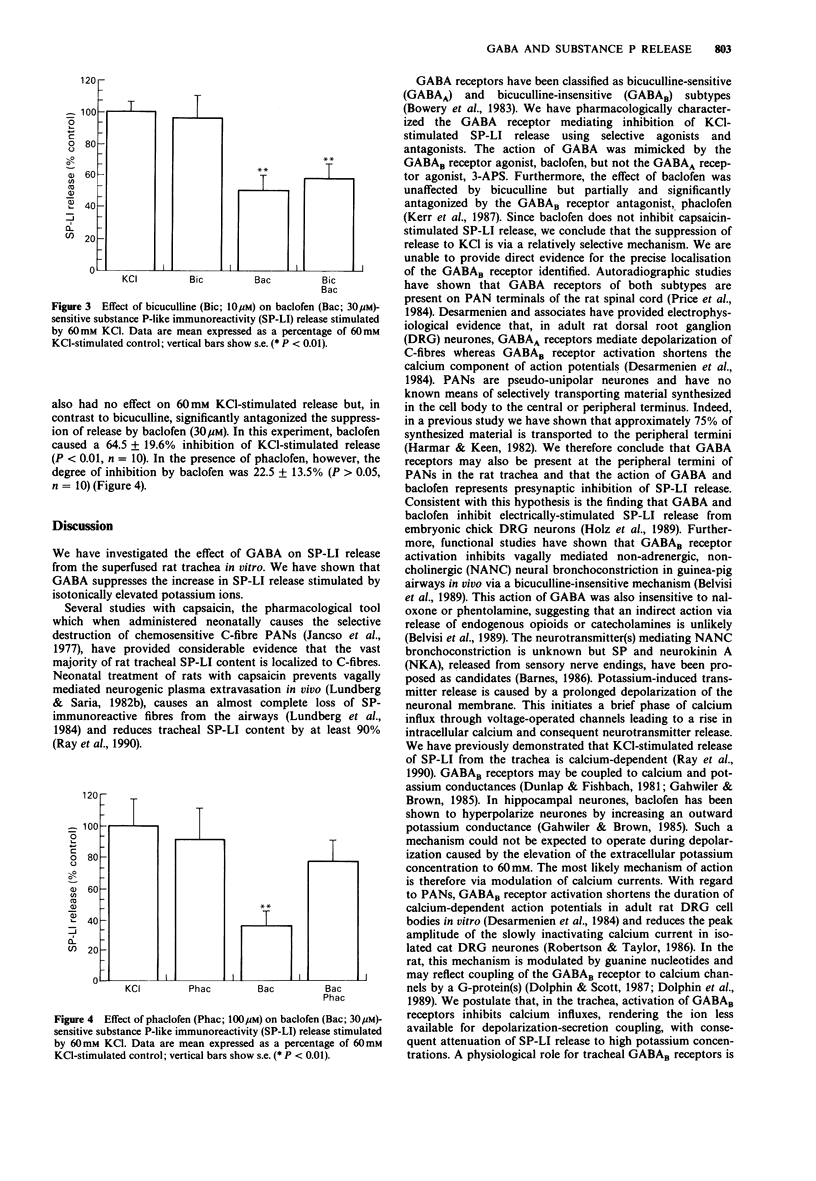
1. The role of gamma-aminobutyric acid (GABA) as an inhibitory transmitter in the central nervous system is well documented. Recently, GABAA and GABAB receptors have been identified in the peripheral nervous system, notably on primary afferent neurones (PAN). We have utilised a multi-superfusion system to investigate the effect of selective GABA receptor agonists and antagonists on the release of substance P (SP) from the rat trachea in vitro. 2. GABA (1-100 microM) did not affect spontaneous release of SP-like immunoreactivity (LI) but caused dose-related inhibition of calcium-dependent potassium (60 mM)-stimulated SP-LI release. The greatest inhibition of 77.7 +/- 18.8% was observed at 100 microM. 3. The inhibitory effect of GABA was mimicked by the GABAB receptor agonist, (+/-)-baclofen (1-100 microM), but not the GABAA receptor agonist, 3-amino-1-propane-sulphonic acid (3-APS, 1-100 microM). Baclofen (100 microM) had no effect on SP-LI release stimulated by capsaicin (1 microM). 4. The inhibitory effect of baclofen (30 microM) was significantly reduced by prior and concomitant exposure to the GABAB receptor antagonist, phacolofen (100 microM) but not the GABAA receptor antagonist, bicuculline (10 microM). Neither antagonist, alone, affected spontaneous or potassium-stimulated SP-LI release. 5. We conclude that activation of pre-synaptic GABAB receptors on the peripheral termini of PANs in the rat trachea inhibits SP-LI release and suggest that GABAB receptor agonists may be of value in the therapeutic treatment of asthma.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J. Asthma as an axon reflex. Lancet. 1986 Feb 1;1(8475):242–245. doi: 10.1016/s0140-6736(86)90777-4. [DOI] [PubMed] [Google Scholar]
- Belvisi M. G., Ichinose M., Barnes P. J. Modulation of non-adrenergic, non-cholinergic neural bronchoconstriction in guinea-pig airways via GABAB-receptors. Br J Pharmacol. 1989 Aug;97(4):1225–1231. doi: 10.1111/j.1476-5381.1989.tb12582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTANTINE J. W. THE SPIRALLY CUT TRACHEAL STRIP PREPARATION. J Pharm Pharmacol. 1965 Jun;17:384–385. doi: 10.1111/j.2042-7158.1965.tb07688.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., McGuirk S. M., Scott R. H. An investigation into the mechanisms of inhibition of calcium channel currents in cultured sensory neurones of the rat by guanine nucleotide analogues and (-)-baclofen. Br J Pharmacol. 1989 May;97(1):263–273. doi: 10.1111/j.1476-5381.1989.tb11950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Calcium channel currents and their inhibition by (-)-baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol. 1987 May;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (-)-baclofen. Br J Pharmacol. 1986 May;88(1):213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désarmenien M., Feltz P., Occhipinti G., Santangelo F., Schlichter R. Coexistence of GABAA and GABAB receptors on A delta and C primary afferents. Br J Pharmacol. 1984 Feb;81(2):327–333. doi: 10.1111/j.1476-5381.1984.tb10082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg G. E., Foster A. C. Amino acid neurotransmitters and their pathways in the mammalian central nervous system. Neuroscience. 1983 Aug;9(4):701–719. doi: 10.1016/0306-4522(83)90263-4. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar A. J., Keen P. M. Methods for the identification of neuropeptide processing products: somatostatin and the tachykinins. Methods Enzymol. 1986;124:335–348. doi: 10.1016/0076-6879(86)24025-2. [DOI] [PubMed] [Google Scholar]
- Harmar A., Keen P. Synthesis, and central and peripheral axonal transport of substance P in a dorsal root ganglion-nerve preparation in vitro. Brain Res. 1982 Jan 14;231(2):379–385. doi: 10.1016/0006-8993(82)90374-2. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Kream R. M., Spiegel A., Dunlap K. G proteins couple alpha-adrenergic and GABAb receptors to inhibition of peptide secretion from peripheral sensory neurons. J Neurosci. 1989 Feb;9(2):657–666. doi: 10.1523/JNEUROSCI.09-02-00657.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X. Y., Theodorsson-Norheim E., Brodin E., Lundberg J. M., Hökfelt T. Multiple tachykinins (neurokinin A, neuropeptide K and substance P) in capsaicin-sensitive sensory neurons in the guinea-pig. Regul Pept. 1985 Dec;13(1):1–19. doi: 10.1016/0167-0115(85)90082-5. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Hills J. M., Saffrey M. J. Immunohistochemical demonstration of GABAergic neurons in the enteric nervous system. J Neurosci. 1986 Jun;6(6):1628–1634. doi: 10.1523/JNEUROSCI.06-06-01628.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Martling C. R., Saria A., Cuello C. Substance P-immunoreactive sensory nerves in the lower respiratory tract of various mammals including man. Cell Tissue Res. 1984;235(2):251–261. doi: 10.1007/BF00217848. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Martling C. R., Saria A., Folkers K., Rosell S. Cigarette smoke-induced airway oedema due to activation of capsaicin-sensitive vagal afferents and substance P release. Neuroscience. 1983 Dec;10(4):1361–1368. doi: 10.1016/0306-4522(83)90117-3. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Bronchial smooth muscle contraction induced by stimulation of capsaicin-sensitive sensory neurons. Acta Physiol Scand. 1982 Dec;116(4):473–476. doi: 10.1111/j.1748-1716.1982.tb07170.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-sensitive vagal neurons involved in control of vascular permeability in rat trachea. Acta Physiol Scand. 1982 Aug;115(4):521–523. doi: 10.1111/j.1748-1716.1982.tb07116.x. [DOI] [PubMed] [Google Scholar]
- McDonald D. M., Mitchell R. A., Gabella G., Haskell A. Neurogenic inflammation in the rat trachea. II. Identity and distribution of nerves mediating the increase in vascular permeability. J Neurocytol. 1988 Oct;17(5):605–628. doi: 10.1007/BF01260989. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Price G. W., Wilkin G. P., Turnbull M. J., Bowery N. G. Are baclofen-sensitive GABAB receptors present on primary afferent terminals of the spinal cord? Nature. 1984 Jan 5;307(5946):71–74. doi: 10.1038/307071a0. [DOI] [PubMed] [Google Scholar]
- Ray N. J., Jones A. J., Keen P. Morphine, but not sodium cromoglycate, modulates the release of substance P from capsaicin-sensitive neurones in the rat trachea in vitro. Br J Pharmacol. 1991 Apr;102(4):797–800. doi: 10.1111/j.1476-5381.1991.tb12254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Taylor W. R. Effects of gamma-aminobutyric acid and (-)-baclofen on calcium and potassium currents in cat dorsal root ganglion neurones in vitro. Br J Pharmacol. 1986 Dec;89(4):661–672. doi: 10.1111/j.1476-5381.1986.tb11170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J., Matsas R., Kenny A. J. Are there neuropeptide-specific peptidases? Biochem Pharmacol. 1985 May 1;34(9):1347–1356. doi: 10.1016/0006-2952(85)90669-0. [DOI] [PubMed] [Google Scholar]


