Abstract
1. Single smooth muscle cells were isolated from bovine trachealis by enzymic digestion. The properties of large conductance plasmalemmal K(+)-channels in these cells were studied by the patch-clamp recording technique. 2. Recordings were made from inside-out plasmalemmal patches when [K+] was symmetrically high (140 mM) and when [Ca2+] on the cytosolic side of the patch was varied from nominally zero to 10 microM. Large unitary currents of both Ca(2+)-dependent and -independent types were observed. Measured between + 20 and + 40 mV, the slope conductances of the channels carrying these currents were 249 +/- 18 pS and 268 +/- 14 pS respectively. 3. Lowering [K+] on the cytosolic side of the patches from 140 to 6 mM, shifted the reversal potentials of the two types of unitary current from approximately zero to much greater than + 40 mV, suggesting that both currents were carried by K(+)-channels. 4. The Ca(2+)-dependent and -independent K(+)-channels detected in inside-out plasmalemmal patches could also be distinguished on the basis of their sensitivity to inhibitors (tetraethylammonium (TEA), 1-10 mM; Cs+, 10 mM; Ba2+, 1-10 mM; quinidine, 100 microM) applied to the cytosolic surface of the patches. 5. Recordings were made from outside-out plasmalemmal patches when [K+] was symmetrically high (140 mM) and when [Ca2+] on the cytosolic side of the patch was varied from nominally zero to 1 microM. Ca(2+)-dependent unitary currents were observed and the slope conductance of the channel carrying these currents was 229 +/- 5 pS.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


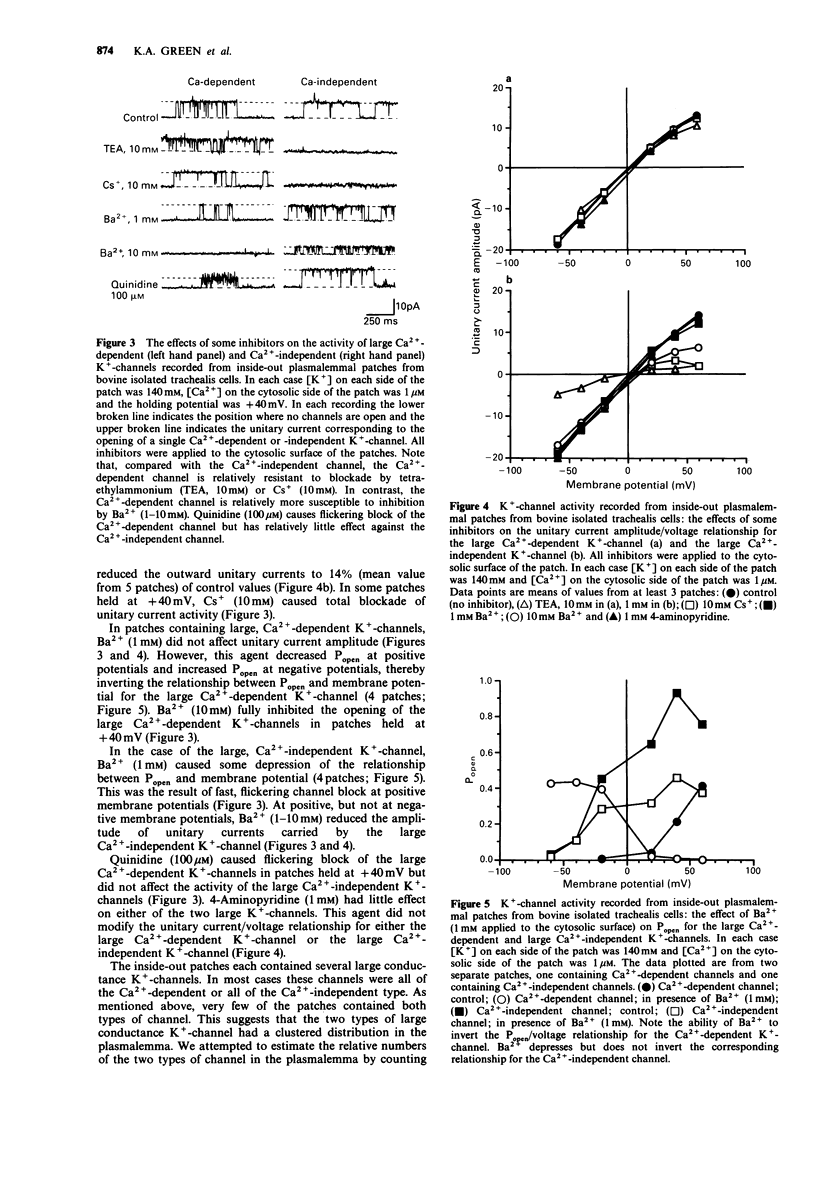
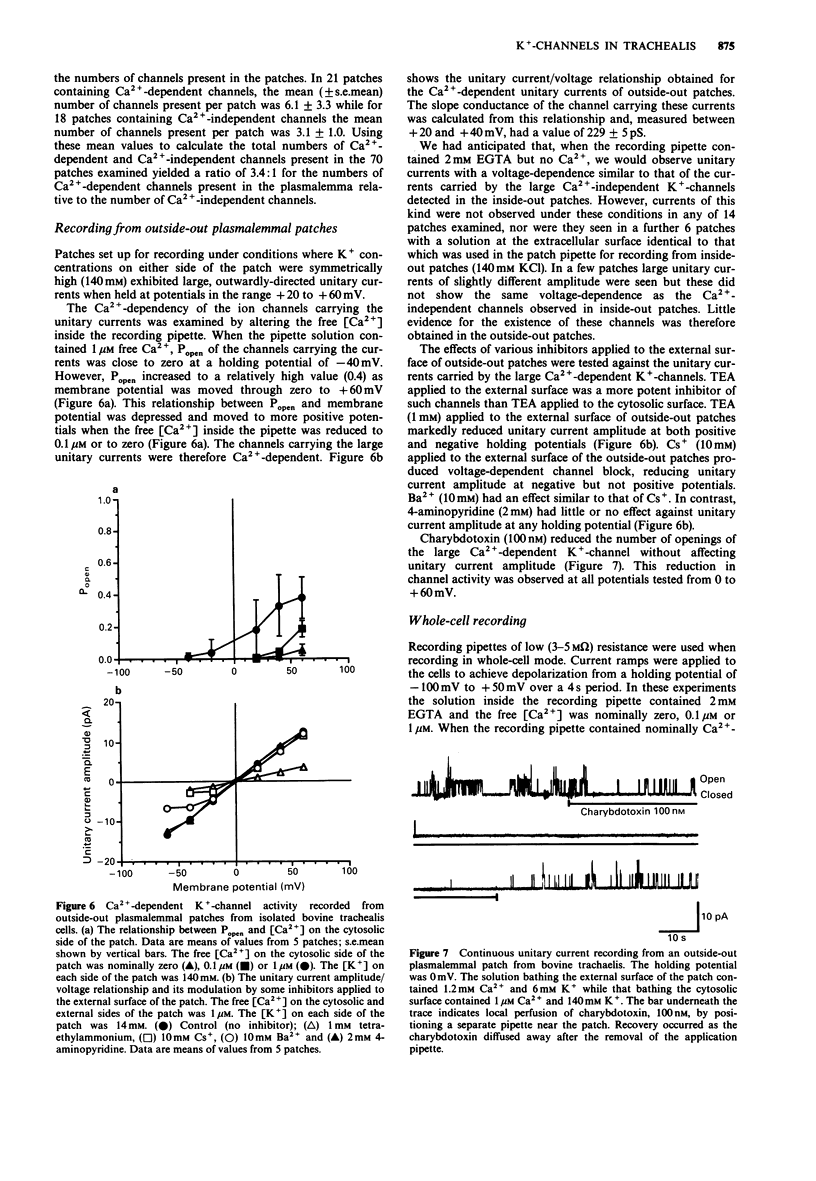
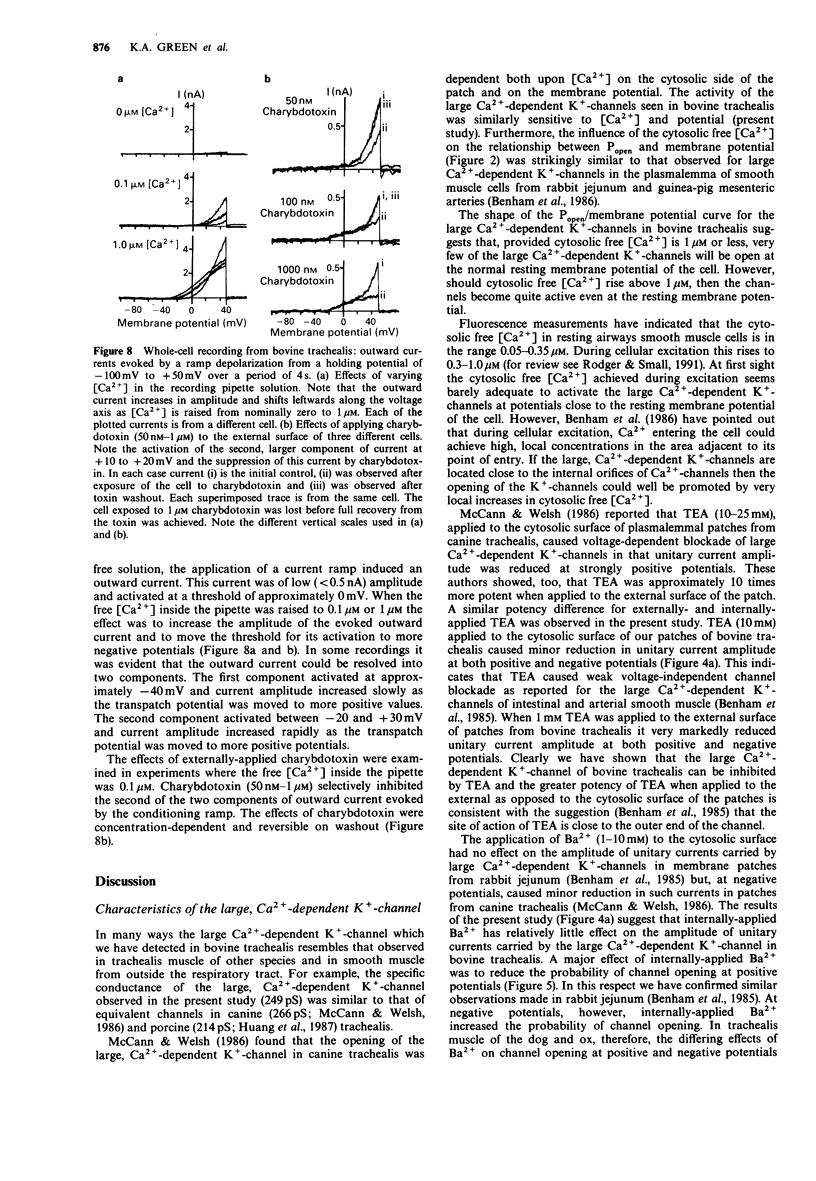


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Patch-clamp studies of slow potential-sensitive potassium channels in longitudinal smooth muscle cells of rabbit jejunum. J Physiol. 1983 Jul;340:469–486. doi: 10.1113/jphysiol.1983.sp014774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Hisada T., Kurachi Y., Sugimoto T. Properties of membrane currents in isolated smooth muscle cells from guinea-pig trachea. Pflugers Arch. 1990 Apr;416(1-2):151–161. doi: 10.1007/BF00370237. [DOI] [PubMed] [Google Scholar]
- Kannan M. S., Jager L. P., Daniel E. E., Garfield R. E. Effects of 4-aminopyridine and tetraethylammonium chloride on the electrical activity and cable properties of canine tracheal smooth muscle. J Pharmacol Exp Ther. 1983 Dec;227(3):706–715. [PubMed] [Google Scholar]
- Kroeger E. A., Stephens N. L. Effect of tetraethylammonium on tonic airway smooth muscle: initiation of phasic electrical activity. Am J Physiol. 1975 Feb;228(2):633–636. doi: 10.1152/ajplegacy.1975.228.2.633. [DOI] [PubMed] [Google Scholar]
- Kume H., Takagi K., Satake T., Tokuno H., Tomita T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J Physiol. 1990 May;424:445–457. doi: 10.1113/jphysiol.1990.sp018076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki K., Imaizumi Y., Kojima T., Kawai T., Watanabe M. Effects of tetraethylammonium and 4-aminopyridine on outward currents and excitability in canine tracheal smooth muscle cells. Br J Pharmacol. 1990 Jul;100(3):507–515. doi: 10.1111/j.1476-5381.1990.tb15838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C., Phillips M., Miller C. Purification of charybdotoxin, a specific inhibitor of the high-conductance Ca2+-activated K+ channel. J Biol Chem. 1986 Nov 5;261(31):14607–14613. [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Morita K., Kuriyama H. Innervation and properties of the smooth muscle of the dog trachea. Jpn J Physiol. 1976;26(3):303–320. doi: 10.2170/jjphysiol.26.303. [DOI] [PubMed] [Google Scholar]


