Abstract
Cyclophilins are a family of proteins that bind cyclosporin A (CsA) and possess peptidyl-prolyl cis-trans isomerase activity. In addition, they are secreted by activated cells and act in a cytokine-like manner, presumably via signaling through a cell surface cyclophilin receptor. More recently, host-derived cyclophilin A (CyPA) has been shown to be incorporated into HIV-1 virions and its incorporation essential for viral infectivity. Here we present evidence supporting a role for viral-associated CyPA in the early events of HIV-1 infection. We report that HIV-1 infection of primary peripheral blood mononuclear cells can be inhibited by: (i) an excess of exogenously added CyPA; (ii) a CsA analogue unable to enter the cells; (iii) neutralizing antibodies to CyPA. Taken together with our observations that recombinant CyPA-induced mobilization of calcium in immortalized, as well as primary, CD4+ T lymphocytes, and that incubation of T cells with iodinated CyPA, followed by chemical cross-linking, resulted in the formation of a high molecular mass complex on the cell surface, these results suggest that HIV-1-associated CyPA mediates an early event in viral infection via interaction with a cellular receptor. This interaction may present a target for anti-HIV therapies and vaccines.
The finding that cyclophilin A (CyPA), a cellular protein that binds cyclosporin A (CsA) and possesses peptidyl-prolyl cis-trans isomerase activity (1, 2), is specifically incorporated into HIV-1 virions through interactions with the Gag polyprotein (3, 4) and is required for effective infection (5–8) raised a number of interesting questions about the biology of this phenomenon. Indeed, although viruses are known to use cellular proteins for their benefit, CyPA is the first such protein found to be incorporated into the virion during virus assembly and to perform an important function in the next cycle of viral infection. Because cyclophilins can control protein folding by catalyzing peptidyl-prolyl isomerization (9, 10), it has been proposed that CyPA may modify the conformation of capsid antigen (CA), a Gag polyprotein cleavage product that binds CyPA (11), thus promoting uncoating of the virion and formation of the pre-integration complex (PIC) (6). Consistent with this hypothesis, formation of the functional HIV-1 PIC is associated with dissociation of CA (12, 13), likely soon after the fusion process.
However, it has not been established yet whether the function of CyPA in HIV-1 infection is accomplished through a direct effect on CA (or other viral proteins), or involves additional cellular proteins on a target cell. This latter possibility is suggested by the finding that CyPA resembles cytokines in two important aspects: it is released into the culture medium by lipopolysaccharide-stimulated macrophages (14), and it has proinflammatory (15) and leukocyte chemotactic activity (16). These features suggest that leukocytes might express a cell-surface receptor for CyPA. In this report we provide evidence that interaction between virion-associated CyPA and this putative CyPA-binding receptor is critical for establishing productive HIV-1 infection of target cells.
MATERIALS AND METHODS
Cloning and Expression of Murine CyPA.
One microgram of lipopolysaccharide-stimulated murine macrophage RAW 264.7 total RNA was reverse transcribed, and the resulting cDNA amplified by PCR by using a set of cyclophilin-specific primers (5′-CCATGGTCAACCCCACC-3′ and 5′-ACGCTCTCCTGAGCTACAGA-3′) that span the murine cyclophilin (cyclophilin A) coding region. The resultant DNA fragment was cloned into the pET14b vector (Novagen), and expressed as a histidine fusion protein. The histidine-tagged CyPA was eluted with 1 M imidazole, and the histidine-tagged leader sequence was removed by proteolytic cleavage with 0.5 units of thrombin per mg recombinant protein. The mixture was then concentrated and purified by FPLC gel filtration chromatography over a Superose 12 column (Pharmacia). Cyclophilin eluted as a sharp peak with an apparent molecular mass of 19 kDa. The material was >98% pure as judged from silver-stained SDS/polyacrylamide gels.
Calcium Mobilization Assay.
Cells were washed and incubated at 37°C for 30 min with 5 μM Fura-2/AM (Calbiochem) at 107 cells per ml in Hanks’ balanced salt solution (2 mM CaCl2/145 mM NaCl/5 mM KCl/1 mM MgSO4/10 mM D-glucose/10 mM Hepes, pH 7.4) containing 1% fetal bovine serum and 10 μM Probenecid (Sigma). After the initial loading period, the cell suspensions were diluted 3-fold with Hanks’ balanced salt solution without CaCl2 and incubated for 10 min at 37°C to ensure complete hydrolysis by intracellular esterase of Fura-2/AM. Cells were then spun down and resuspended in Hanks’ balanced salt solution without serum at 5 × 106 cells per ml. The cells were prewarmed at 37°C for 20 min and transferred into an acrylic cuvette. Fluorescence measurements of intracellular Ca2+ were performed on a Perkin–Elmer Luminescence Spectrometer LS50B at an emission wavelength of 500 nm and a frequency of 1.6 Hz, following excitation at 340 and 380 nm. Final [Ca2+]i values were calculated from the ratio of emission at 340/380 nm excitation according to the standard equation by using dissociation constant of 224 nM for Fura-2.
Advanced Glycosylation Endproduct (AGE) Modification of Murine Cyclophilin.
An aliquot of purified recombinant murine cyclophilin (500 μg/ml final) was reacted with glucose (1 M final) in 0.5 M sodium phosphate buffer (pH 7.4) containing 1 mM EDTA for three months at 37°C in a nonhumidified air incubator. Upon completion, the reaction mixture was dialyzed to remove noncovalently attached low molecular mass reactants, aliquoted, and stored frozen at −20°C until used for immunization of rabbits (17).
Generation of Rabbit Anti-Cyclophilin and Anti-AGE-Modified Cyclophilin Antisera.
Female New Zealand white rabbits were immunized with either cyclophilin (50 μg) or AGE-modified cyclophilin (125 μg) emulsified in 1.0 ml saline plus 1.0 ml complete Freund’s adjuvant. Animals were administered booster injections monthly with the same antigen emulsified in 1.0 ml saline and 1.0 ml incomplete Freund’s adjuvant. Blood was withdrawn 7 days and 14 days following each booster injection, and the serum fraction was recovered and stored frozen at −20°C.
Affinity Purification of Anti-Cyclophilin Serum.
Five milliliters of polyclonal rabbit anti-AGE-CyPA serum that had been dialyzed overnight against 10 mM Tris (pH 7.5) was loaded onto a column prepared by coupling recombinant murine CyPA to Sepharose-4B. Cyclophilin-specific antibodies were eluted with 100 mM glycine⋅HCl (pH 2.5). One ml fractions were collected and pH was adjusted to 7.4 by the addition of 1 M Tris (pH 9.0). Peak fractions were pooled, dialyzed against 0.05 M sodium phosphate (pH 7.0), and then further fractionated by using protein A Sepharose chromatography to purify Ig fraction.
Preparation of CsA-Poly(Ethylene Glycol) (PEG).
The CsA analog, d-diaminopropionic8(tert-butyloxycarbonyl)-CsA (18) was deprotected and the resulting 8-amino-CsA covalently attached to PEG according to previously established procedures (19, 20). To prepare the control reagent (X-PEG), the reaction was performed identically with the exception that 8-amino-CsA was omitted. Immediately prior to use, aliquots of CsA-PEG and X-PEG were diluted into ethanol and remaining traces of methylene chloride were removed by blowing a slow stream of nitrogen over the sample.
Cross-Linking of 125I-Labeled Murine Cyclophilin to Membrane Proteins on H9 Cells.
Recombinant murine CyPA was iodinated by using the Bolton-Hunter reagent (NEN) according to manufacturer’s instructions. The resulting reagent had a specific activity of 1.6 × 102 μCi/μmol. H9 cells were harvested, washed in ice-cold PBS, and 1.25 × 107 cells were transferred to a clean tube. The cells then were pelleted by centrifugation and resuspended in 0.5 ml ligand binding medium (RPMI 1640 medium supplemented with 25 mM Hepes and 1% BSA), and 100 nmol of 125I-cyclophilin (either alone, or in combination with a 100-fold excess of cold cyclophilin) was added. After a 60-min incubation on ice, cells were pelleted at 500 × g, and supernatant discarded. The pellet was resuspended to 1.25 × 106 cells per ml in cross-linking buffer (PBS supplemented with 1 mM MgCl2), and dithiobis(succinimidylpropionate) was added to a final concentration of 2 mM. The reaction mixture was incubated at 16°C for 20 min, at which time 1 volume of TE buffer (50 mM Tris⋅HCl, pH 7.4/1 mM EDTA) was added to stop the reaction. Cells were washed three times in ice-cold TE buffer, and then extracted in lysis buffer (50 mM Tris⋅HCl, pH 7.6/300 mM NaCl/0.5% Triton X-100). After centrifugation, the lysates were analyzed by SDS/PAGE electrophoresis and autoradiography.
Viruses, Cells, and Infection.
The T-lymphotropic strain HIV-1RF (21) was used to infect peripheral blood mononuclear cells (PBMCs), and the macrophage-tropic HIV-1ADA (22) was used for infection of primary macrophages. Macrophage and PBMC cultures were prepared as described elsewhere (H. Schmidtmayerova, M. Alfano, G. Nuovo, and M. Bukrinsky, unpublished data). Prior to infection, viral stocks were treated with 200 units/ml of RNase-free DNase for 1 hr at room temperature to eliminate contaminating virus-specific DNA. Cells were infected for 2 hr at 37°C with an amount of virus corresponding to 1.5 × 105 cpm/ml of reverse transcriptase activity per 1 × 106 cells. After infection, macrophages were treated with Trypsin-EDTA (0.05% Trypsin/0.53 mM EDTA, GIBCO/BRL) for 5–10 min at 37°C. Trypsin treatment was stopped by the addition of medium containing 10% human serum, and cells were then washed with serum-free DMEM.
PCR Analysis of Infection.
PCR analysis of HIV-1 DNA and RNA was performed as described elsewhere (H. Schmidtmayerova, M. Alfano, G. Nuovo, and M. Bukrinsky, unpublished data). Dilutions of 8E5/LAI cells (23) containing one HIV-1 provirus per cell were used as standards.
RESULTS
Exogenous CyPA Inhibits HIV-1 Infection.
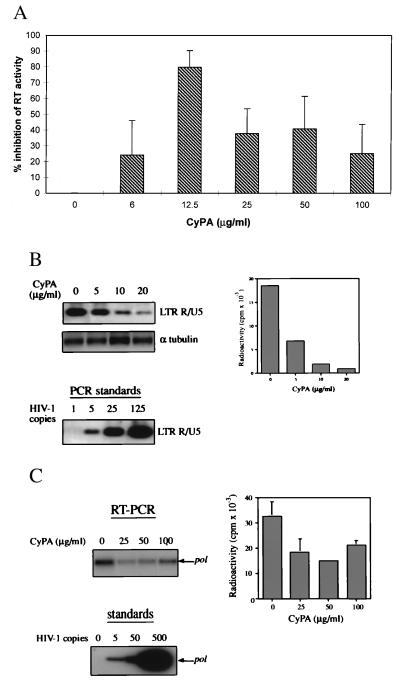
Primary PBMCs were infected with the HIV-1RF strain in the presence of various concentrations of recombinant CyPA, and viral replication was monitored by the reverse transcriptase (RT) activity in culture supernatants. To account for the well-documented donor-dependent variability in infectivity of PBMCs, experiments were repeated on PBMCs from six different donors, each performed in triplicate, and the results were tabulated as the percent inhibition of RT activity in CyPA-treated versus untreated (control) cultures from the same donor. Exogenously added CyPA was found to inhibit HIV-1 replication, with the maximal inhibitory effect achieved at CyPA concentrations 12.5 μg/ml (Fig. 1A). Surprisingly, higher concentrations of CyPA gave less inhibition, resulting in a bell-shaped dose response curve (Fig. 1A). The level of HIV-1 suppression in culture is a cumulative result of multiple events during many rounds of viral replication. Thus, this experiment did not allow us to identify any specific step of viral replication affected by exogenous addition of CyPA. Because previous studies suggested that virion-associated CyPA is involved in a step of infection following binding of the virus to the cell but preceding reverse transcription (6), we analyzed the effect of exogenous CyPA on virus entry. For this experiment, we used CyPA in concentrations close to the concentration (12.5 μg/ml) giving maximal inhibitory effect in a long-term culture. Early products of reverse transcription were assayed by a quantitative PCR technique 2 hr after infection of T lymphocytes. Amplification of a set of standards with known concentrations of the HIV-1 template was performed in parallel to establish the relationship between the amount of the template and the quantity of the amplified product (Fig. 1B, Lower Left). Tubulin DNA was amplified to normalize for the concentration of DNA in each lysate. The presence of CyPA during infection reduced in a dose-dependent manner the amount of HIV-1 cDNA present as the early product of reverse transcription (Fig. 1B, Upper Left). The amount of radioactivity in the bands was quantified on the Packard Direct Imager (Fig. 1B, Right). A 80–90% reduction in the amount of viral DNA was observed in cells treated with 10–20 μg/ml of CyPA. Because PCR primers used in this reaction amplify an early product of reverse transcription synthesized before the first strong-stop (24), the detected HIV-1-specific fragment reflects mostly viral DNA that entered the cell with the infecting virus (25). In support of this was the observation that the synthesis of this product was partially resistant to 3′-azido-3′-deoxythymidine inhibition (not shown). This result, therefore, indicated that exogenous CyPA interfered at least in part with early events in the entry of the virus. To directly test this conclusion, and also to demonstrate the role of CyPA in a different virus-cell system, we measured viral RNA uptake by macrophages infected with a macrophage-tropic strain HIV-1ADA. Two hours after infection, cells were washed and treated with trypsin to remove virus that had not entered the cells. Intracellular HIV-1 RNA was then detected by RT-PCR. As shown in the left panel of Fig. 1C, CyPA reduced in a dose-dependent manner the amount of intracellular viral RNA. We did not perform a long-term analysis of HIV-1 replication in CyPA-treated macrophage cultures, because small lipopolysaccharide contaminations in recombinant CyPA could affect the result. Results of two experiments (with the cells from the same donor) were quantified on a Packard Direct Imager and are shown in Fig. 1C (Right). A 55–60% reduction in the amount of intracellular viral RNA by 25–50 μg/ml of CyPA was observed, suggesting that inhibition of HIV-1 entry by exogenous CyPA might involve competition against virus-associated CyPA for target cell CyPA binding sites.
Figure 1.
Recombinant CyPA inhibits HIV-1 infection. (A) PBMCs from 6 different donors were infected with HIV-1RF in the presence of indicated concentrations of recombinant murine CyPA. At the peak of viral infection (typically day 7 to 11 after infection), RT activity was measured in culture supernatants. The results were tabulated as percentage of inhibition relative to untreated HIV-infected cultures from the same donor. The data are mean ± SEM across donors. (B) PBMCs were infected with HIV-1RF in the presence of indicated concentrations of recombinant murine CyPA. Two hours after infection, cells were washed and viral DNA was analyzed by PCR by using primers that amplify the R/U5 region of the LTR. Amplified fragments were revealed by Southern hybridization, and the amount of product was quantified on Packard Direct Imager (bar graph on the right). A fragment of the α tubulin gene was amplified from the same samples to control for possible differences in the amount of total DNA. Dilutions of 8E5 cells containing the indicated number of HIV-1 genomes were amplified in parallel as standards. Results are shown for one representative experiment out of two performed. (C) Primary differentiated human macrophages were infected with HIV-1ADA in the presence of the indicated concentrations of CyPA. Two hours after infection, cells were washed and treated with trypsin. Viral RNA then was analyzed by RT-PCR by using primers specific for the pol gene. Results of one out of two independent experiments are shown on the left. Results of both experiments were quantified on a Packard Direct Imager and plotted on the right as mean ± SE. Dilutions of 8E5 cells were amplified in parallel, as above, to provide a standard curve.
CyPA-Binding Sites on CD4+ T Lymphocytes.
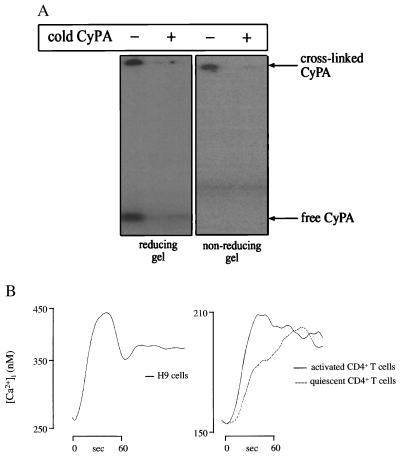
To demonstrate the presence of CyPA-binding sites on CD4+ T lymphocytes, we incubated iodinated CyPA with H9 cells (an immortalized CD4+ T cell line) in the absence or presence of a 100-fold excess of cold CyPA as competitor. After cross-linking of the surface molecules with dithiobis(succinimidylpropionate), proteins were extracted with Triton X-100 and analyzed by SDS/PAGE and autoradiography. Iodinated CyPA was associated with a high-molecular mass band that did not migrate out of the stacking gel under nonreducing conditions (Fig. 2A, Right). Association of 125I-CyPA with this high-molecular mass complex was blocked by a 100-fold excess of cold CyPA. When proteins were analyzed by reducing SDS/PAGE (Fig. 2A, Left), free CyPA was released from the complex. These results suggested that CyPA binds to a complex structure on the surface of CD4+ T lymphocytes.
Figure 2.
Cyclophilin-binding sites on CD4+ T lymphocytes. (A) Iodinated CyPA was added to H9 cells in the presence or absence of a 100-fold excess of cold CyPA. After cross-linking with a thiol-cleavable reagent, dithiobis(succinimidylpropionate), cells were extracted with Triton X-100 to solubilize membrane proteins, and the extract was analyzed by PAGE (10–15%) under either reducing (Left) or nonreducing (Right) conditions. Note that the high molecular mass complex did not migrate out of the stacking gel. (B) Analysis of intracellular calcium was performed on H9 cells (Left graph) or on purified CD4+ T cells, either quiescent or activated with phytohemagglutinin and IL-2 (Right graph).
To determine whether CyPA binding initiated intracellular signal transduction in T cells, we analyzed intracellular calcium flux. Addition of 400 μg/ml of recombinant murine CyPA induced a specific flux of intracellular Ca2+ in both H9 cells (Fig. 2B, Left) and in primary CD4+ T lymphocytes (Fig. 2B, Right). Interestingly, both quiescent and phytohemagglutinin plus interleukin 2-activated CD4+ T cells responded to CyPA (Fig. 2B, Right), indicating that the receptor for CyPA is not regulated upon T cell activation. Similar results were obtained with human CyPA (not shown), which differs only in three amino acids from its mouse homologue (26).
HIV-1 Infection Can Be Inhibited by Extracellular CsA.
The interaction between virion-associated CyPA and a CyPA-binding protein on a target cell may occur either during or after the fusion of viral and cellular membranes. In the first scenario, virion-associated CyPA may become exposed at some point during the process of virus-cell interaction. To test this possibility, we used polyethyleneglycol-modified CsA (CsA-PEG, Fig. 3A). Addition of a bulky side group to CsA blocked entry of this compound into T cells, as revealed by the lack of inhibition of T cell proliferation (Fig. 3B). Nevertheless, CsA-PEG could still partially inhibit HIV-1 infection of T cells (Fig. 3C). Although not complete, inhibition by CsA-PEG was similar to that by unmodified CsA (Fig. 3C), suggesting that virus-associated CyPA becomes accessible for extracellular interaction with CsA-PEG during virus-cell interaction.
Figure 3.
Extracellular CsA inhibits HIV-1 infection. (A) Synthesis of polyethyleneglycol-modified CsA (b) from 8-amino-CsA (a), an analogue of CsA that retains its immunosuppressive activity and ability to inhibit cis-trans isomerase activity of CyPA (19). (B) Incorporation of [3H]thymidine into T cells was measured in the presence of indicated concentrations of CsA, CsA-PEG, or X-PEG (control for CsA-PEG). (C) PBMCs were infected with HIV-1RF in the presence of 1 μg/ml of CsA, CsA-PEG, or X-PEG. Five days after infection, RT in the supernatant was measured. Results are mean ± SD of three independent infections with the cells from the same donor.
Anti-CyPA Antiserum Inhibits HIV-1 Infection.
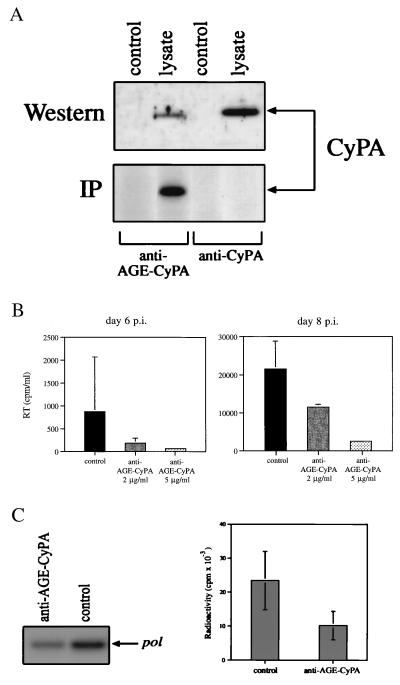
The finding that virion-associated CyPA becomes accessible for extracellular interaction suggested the possibility that HIV-1 infection could be inhibited by antibodies recognizing CyPA. To generate such antibodies, rabbits were immunized either with recombinant mouse CyPA, or with mouse CyPA modified by AGE (AGE-CyPA). The AGE modification strategy was employed as it has been shown to enhance the immunogenicity of conserved proteins (17, 27). Although sera from both rabbits reacted with CyPA from a lysate of murine macrophages in a Western blot assay (Fig. 4A, Upper), only sera from rabbits immunized with AGE-CyPA could immunoprecipitate metabolically labeled murine CyPA (Fig. 4A, Lower). A similar result was obtained with CyPA from human T cells (data not shown). Sera from AGE-CyPA-immunized animals was fractionated on a CyPA affinity resin, and the affinity-purified antibodies were used in blocking experiments. Two concentrations of affinity purified antibodies (2 μg/ml and 5 μg/ml) were added together with HIV-1RF to PBMC cultures and infection was monitored by analysis of viral RT activity in culture supernatants. Anti-AGE-CyPA antibodies inhibited HIV-1 infection in a concentration-dependent manner (Fig. 4B). To determine whether exogenously supplied anti-AGE-CyPA antibodies inhibited viral entry, we analyzed the uptake of HIV-1 RNA in the same manner as for exogenous CyPA, by using primary macrophages infected with HIV-1ADA. Here whole serum from rabbits immunized with AGE-CyPA reduced the amount of intracellular viral RNA by 55% compared with cells treated with preimmune serum (Fig. 4C).
Figure 4.
Antibodies to CyPA inhibit HIV-1 infection. (A). Sera from rabbits immunized with CyPA (anti-CyPA) or with AGE-modified CyPA (anti-AGE-CyPA) was used to reveal CyPA in a Western blot or immunoprecipitation (IP) assay. For Western analysis, a lysate of murine RAW 264.7 cells was fractionated on an SDS/PAGE. For IP analysis, lysate of RAW 264.7 cells metabolically labeled with [35S]methionine was immunoprecipitated with anti-CyPA or anti-AGE-CyPA serum (diluted 1:100) and fractionated on SDS/PAGE. (B) PBMCs were infected with HIV-1RF in the absence (control) or presence of affinity-purified anti-AGE-CyPA Ig. RT was measured in culture supernatants at days 6 and 8 after infection. Results are mean ± SE of three independent infections. (C) Primary human macrophages were infected with HIV-1ADA in the presence of anti-AGE-CyPA serum (1:40 dilution) or pre-immune (control) rabbit serum. Two hours after infection, cells were washed, treated with trypsin, and viral RNA was analyzed by RT-PCR by using pol-specific primers. Results of one out of two independent experiments are shown on the left, and both experiments are quantified on the Packard Direct Imager and plotted on the right as mean ± SE.
DISCUSSION
Results presented in this report demonstrate that HIV-1 infection can be inhibited in vitro by agents targeted to the interaction between virion-associated CyPA and putative CyPA-binding sites on CD4+ T lymphocytes. Two lines of evidence suggest that such putative sites are indeed present on T cells. Firstly, iodinated CyPA binds to T cells, and this binding is competed by excess cold CyPA. Secondly, CyPA initiates signal transduction in T cells as revealed by mobilization of intracellular Ca2+. Although we have not yet characterized the protein(s) to which CyPA binds, this binding activity most likely is located on the cell surface because the cross-linking agent employed does not enter into the cells. The existence of signal-transducing CyPA-binding sites is consistent with the published data reporting the presence of secreted CyP(s) in biological fluids (15, 16) and culture medium supernatants (14, 28), which possess functions usually attributed to cytokines and growth factors (14, 16, 29, 30). A receptor for another member of the cyclophilin family, cyclophilin B (CyPB), which is highly homologous to CyPA, has been recently identified on CD4+ T lymphocytes (31, 32). An earlier report (31) suggested that this receptor is not shared by CyPA. However, a possibility remains that CyPA binds to this receptor with a lower affinity than CyPB, thus explaining the inability of CyPA to compete with CyPB in a surface binding assay (31).
The presence of CyPA-binding sites on CD4+ T cells suggested to us that the process of HIV-1 infection might involve interaction between such sites and virion-associated CyPA. To discriminate between this possibility and the model suggested by others (6, 33, 34) whereby the role of CyPA is to regulate folding of CA during the PIC formation (6, 35), we used an excess of recombinant CyPA to compete with virion-incorporated CyPA for interaction with the putative CyPA-binding site. The observed inhibition of viral replication by exogenous CyPA (Fig. 1) suggested that interaction with a CyPA-binding site was required for infection, because the folding function can presumably be mediated equally well by both exogenous and virion-associated cyclophilins and thus would not be affected by excess exogenous CyPA. The inhibitory effect of added soluble CyPA on HIV-1 replication had an unusual bell-shaped dose response curve (Fig. 1). This suggests that CyPA at higher concentrations may have distinct stimulatory activity on viral replication. Consistent with this explanation is our finding that high concentrations of CyPA induced calcium flux in T cells (Fig. 2B), an indicator of receptor-mediated signaling often associated with cell activation. As T cell activation is associated with high levels of virus production, CyPA at high concentrations may well stimulate HIV-1 replication. This explanation is also consistent with a much higher inhibitory activity of CyPA measured by PCR under one infection cycle conditions (Fig. 1B), when only viral entry is evaluated, than observed in a long-term culture (Fig. 1A).
Our results indicate that agents targeting interaction between viral CyPA and surface-associated cellular CyPA-binding protein(s) reduce HIV-1 entry (Figs. 1 B and C and 4C). Therefore, CyPA-binding sites on target cells appear to function as co-receptors for the virus, in addition to previously identified CD4 and chemokine receptors (reviewed in ref. 36). However, in contrast to classical HIV-1 receptors, dependence of virus-cell fusion on CyPA may not be absolute. Indeed, CyPA did not inhibit fusion in an Env-mediated cell-cell fusion assay (E. Berger, personal communication). A possible explanation for this discrepancy is that virion-associated CyPA serves to accelerate or otherwise enhance virus-cell fusion, but is not indispensable. This interpretation is consistent with the relatively mild inhibition observed in our assays compared with the dramatic effect on viral entry of ligands for chemokine receptors (reviewed in ref. 36).
The effect of CyPA on viral entry does not exclude the possibility of a CyPA-mediated activity at a later step of infection, in particular at the step of virus uncoating and PIC formation. One of the major protein rearrangements associated with the formation of the HIV-1 PIC is dissociation of the CA protein from the viral core (12, 13, 37). Formation of the CA-CyPA-CyPA receptor complex may by itself promote dissociation of CA from the viral core and thus facilitate uncoating and the process of PIC assembly. Consistent with this model, the presence of recombinant CyPA during HIV-1 infection resulted in the same phenotype as that observed with CyPA-deficient virus, i.e., infection was blocked at an early step prior to initiation of reverse transcription. It appears likely that the same CyPA binding site is involved in both entry and postentry steps, thus providing the link between the fusion process and uncoating of HIV-1.
Interaction between the virion-associated CyPA and CyPA binding site may occur either during the fusion step, or after fusion (i.e., inside the target cell). Our finding that PEG-modified CsA (CsA-PEG), which did not have immunosuppressive activity (Fig. 3B) most likely due to its inability to enter the cells, could still inhibit HIV-1 infection (Fig. 3C), suggests that virion-associated CyPA becomes accessible to the extracellular compartment at some point during the fusion process. This is quite a surprising observation, given a demonstrated localization of CyPA inside the virion, presumably within a core structure (5). A possible explanation for this finding may be that CyPA protrudes through the viral envelope in the places of contact between viral envelope and the core (38). Alternatively, CyPA may become partly exposed during the fusion process as a result of perturbations of the viral membrane. Consistent with this explanation, the magnitude of the inhibitory effect of CsA-PEG was less than that of exogenous CyPA (Fig. 3C), indicating that viral CyPA was only partially accessible.
Inhibition of HIV-1 infection by CsA-PEG suggested that a similar effect might be achieved with antibodies to CyPA. Indeed, anti-CyPA antibodies inhibited HIV-1 infection in T cells (Fig. 4). Obtaining neutralizing antibodies that recognize native CyPA was complicated by the high degree of interspecies conservation of cyclophilins (26), leading to immunologic tolerance to native cyclophilins. To circumvent this problem, we used as immunogen CyPA modified by advanced glycation (AGE-CyPA) (17, 27). Glycation is believed to alter antigen processing, such that new epitopes, which are not normally immunogenic, are presented for antibody generation (27). This can lead to antisera that recognize conserved native epitopes, and in this case, antisera against AGE-CyPA immunoprecipitated native, unmodified CyPA (Fig. 4A) and reduced viral infection (Figs. 4 B and C).
The possibility of inhibiting HIV-1 infection by using extracellular CyPA-targeting agents is potentially attractive. Of course, additional studies are required to investigate the dependence of various primary isolates on CyPA and their sensitivity to CyPA-targeting reagents. Although one published report (39) suggested that most HIV-1 clades from group M require CyPA for replication, our analysis demonstrated differential sensitivity of primary HIV-1 isolates to inhibition by CyPA and anti-CyPA antibodies. The primary nonsyncytium-inducing isolate 92US072 was more sensitive than 92US660, and both were less sensitive than an SI virus 92UG021 (L.D. and M.B., unpublished results). In addition, the magnitude of inhibitory effect was cell donor-dependent.
Utilizing virus-associated CyPA as a drug target potentially avoids the problem of viral resistance. Because CyPA is host-derived, escape mutants could not easily develop. Disruption of CyPA packaging into HIV-1 virions by mutations or by CsA renders the virus defective in replication (3, 4, 6). Although mutants resistant to CsA have been obtained (35, 40) and shown to be independent of CyPA, their replication was dependent on the presence of CsA, at least in certain cell types (35). Because virus assembly occurs intracellularly, CsA-PEG would not be expected to be incorporated into HIV-1 virions, and if CsA-resistant mutants would spontaneously arise in vivo, they would still be defective in replication. Targeting CyPA in the HIV-1 virions with cyclosporins having a bulky side-chain would potentially avoid immunosuppressive and other side effects associated with the intracellular activities of CsA.
Our results also suggest an alternative approach to developing an anti-HIV vaccine, by inducing immunity against CyPA. Vaccination might be performed either by passive introduction of anti-CyPA antibodies, or by immunization with AGE-CyPA, as shown in this report. Although little is known about the normal function of extracellular CyPA, the fact that animals used for production of anti-AGE-CyPA antibodies did not show signs of toxicity indicates that under normal conditions such immunity does not have an adverse effect. A more detailed morphologic analysis of organs from such animals, as well as studies of their immune reactions to various infections, would help to determine the safety of this approach and its validity for preventing HIV-1 infection.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 AI 29110 (B.S.) and R01 AI 38245 (M.B.) and by the funds from The Picower Institute for Medical Research.
ABBREVIATIONS
- CyPA
cyclophilin A
- CsA
cyclosporin A
- PIC
pre-integration complex
- CA
capsid antigen
- AGE
advanced glycosylation endproduct
- RT
reverse transcriptase
- PBMC
peripheral blood mononuclear cell
- PEG
poly(ethylene glycol)
References
- 1.Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid F X. Nature (London) 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Ke H. Biochemistry. 1996;35:7356–7361. doi: 10.1021/bi9602775. [DOI] [PubMed] [Google Scholar]
- 3.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh C T, Sodroski J, Gottlinger H G. Nature (London) 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 4.Franke E K, Yuan H E, Luban J. Nature (London) 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 5.Ott D E, Coren L V, Johnson D G, Sowder R C, Arthur L O, Henderson L E. AIDS Res Hum Retroviruses. 1995;11:1003–1006. doi: 10.1089/aid.1995.11.1003. [DOI] [PubMed] [Google Scholar]
- 6.Braaten D, Franke E K, Luban J. J Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billich A, Hammerschmid F, Peichl P, Wenger R, Zenke G, Quesniaux V, Rosenwirth B. J Virol. 1995;69:2451–2461. doi: 10.1128/jvi.69.4.2451-2461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartz S R, Hohenwalter E, Hu M -K, Rich D H, Malkovsky M. Proc Natl Acad Sci USA. 1995;92:5381–5385. doi: 10.1073/pnas.92.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer G, Schmid F X. Biochemistry. 1990;29:2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- 10.Gething M-J, Sambrook J. Nature (London) 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 11.Luban J, Bossolt K L, Franke E K, Kalpana G V, Goff S P. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 12.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller M D, Farnet C M, Bushman F D. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherry B, Yarlett N, Strupp A, Cerami A. Proc Natl Acad Sci USA. 1992;89:3511–3515. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billich A, Winkler G, Aschauer H, Rot A, Peichl P. J Exp Med. 1997;185:975–980. doi: 10.1084/jem.185.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Q, Leiva M C, Fischkoff S A, Handschumacher R E, Lyttle C R. J Biol Chem. 1992;267:11968–11971. [PubMed] [Google Scholar]
- 17.Makita Z, Vlassara H, Cerami A, Bucala R. J Biol Chem. 1992;267:5133–5138. [PubMed] [Google Scholar]
- 18.Nelson P A, Akselband Y, Kawamura A, Su M, Tung R D, Rich D H, Kishore V, Rosborough S L, DeCenzo M T, Livingston D J, et al. J Immunol. 1993;150:2139–2147. [PubMed] [Google Scholar]
- 19.Colucci W J, Tung R D, Petri J A, Rich D H. J Org Chem. 1990;55:242–248. [Google Scholar]
- 20.Haselgrubler T, Amerstorfer A, Schindler H, Gruber H J. Bioconjugate Chem. 1995;6:242–248. doi: 10.1021/bc00033a002. [DOI] [PubMed] [Google Scholar]
- 21.Reitz M S, Jr, Guo H G, Oleske J, Hoxie J, Popovic M, Read-Connole E, Markham P, Streicher H, Gallo R C. AIDS Res Hum Retroviruses. 1992;8:1731. [PubMed] [Google Scholar]
- 22.Gendelman H E, Orenstein J M, Martin M A, Ferrua C, Mitra R, Phipps T, Wahl L A, Lane H C, Fauci A S, Burke D S. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folks T M, Powell D, Lightfoote M, Koenig S, Fauci A S, Benn S, Rabson A, Daugherty D, Gendelman H E, Hoggan M D. J Exp Med. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubei A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. Nature (London) 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trono D. J Virol. 1992;66:4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galat A. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 27.Cerami, A., Vlassara, H., Bucala, R. J. & Skolnik, E. W. (1993) Int. Patent Appl. PCT/US 93/04624.
- 28.Davis T R, Tabatabai L, Bruns K, Hamilton R T, Nilsen-Hamilton M. Biochim Biophys Acta. 1991;1095:145–152. doi: 10.1016/0167-4889(91)90077-b. [DOI] [PubMed] [Google Scholar]
- 29.Caroni P, Rothenfluh A, McGlynn E, Schneider C. J Biol Chem. 1991;266:10739–10742. [PubMed] [Google Scholar]
- 30.Spik G, Haendler B, Delmas O, Mariller C, Chamoux M, Maes P, Tartar A, Montreuil J, Stedman K, Kocher H P, et al. J Biol Chem. 1991;266:10735–10738. [PubMed] [Google Scholar]
- 31.Allain F, Denys A, Spik G. J Biol Chem. 1994;269:16537–16540. [PubMed] [Google Scholar]
- 32.Denys A, Allain F, Foxwell B, Spik G. Immunology. 1997;91:609–617. doi: 10.1046/j.1365-2567.1997.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinkasserer A, Harrison R, Billich A, Hammerschmid F, Werner G, Wolff B, Peichl P, Palfi G, Schnitzel W, Mlynar E, et al. J Virol. 1995;69:814–824. doi: 10.1128/jvi.69.2.814-824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bukovsky A A, Weimann A, Accola M A, Gottlinger H G. Proc Natl Acad Sci USA. 1997;94:10943–10948. doi: 10.1073/pnas.94.20.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braaten D, Aberham C, Franke E K, Yin L, Phares W, Luban J. J Virol. 1996;70:5170–5176. doi: 10.1128/jvi.70.8.5170-5176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger, E. A. (1997) AIDS 11 (Suppl. A), S3–S16. [PubMed]
- 37.Gallay P, Swingler S, Aiken C, Trono D. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 38.Gelderblom H R. AIDS. 1991;5:617–637. [PubMed] [Google Scholar]
- 39.Braaten D, Franke E K, Luban J. J Virol. 1996;70:4220–4227. doi: 10.1128/jvi.70.7.4220-4227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aberham C, Weber S, Phares W. J Virol. 1996;70:3536–3544. doi: 10.1128/jvi.70.6.3536-3544.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]