Abstract
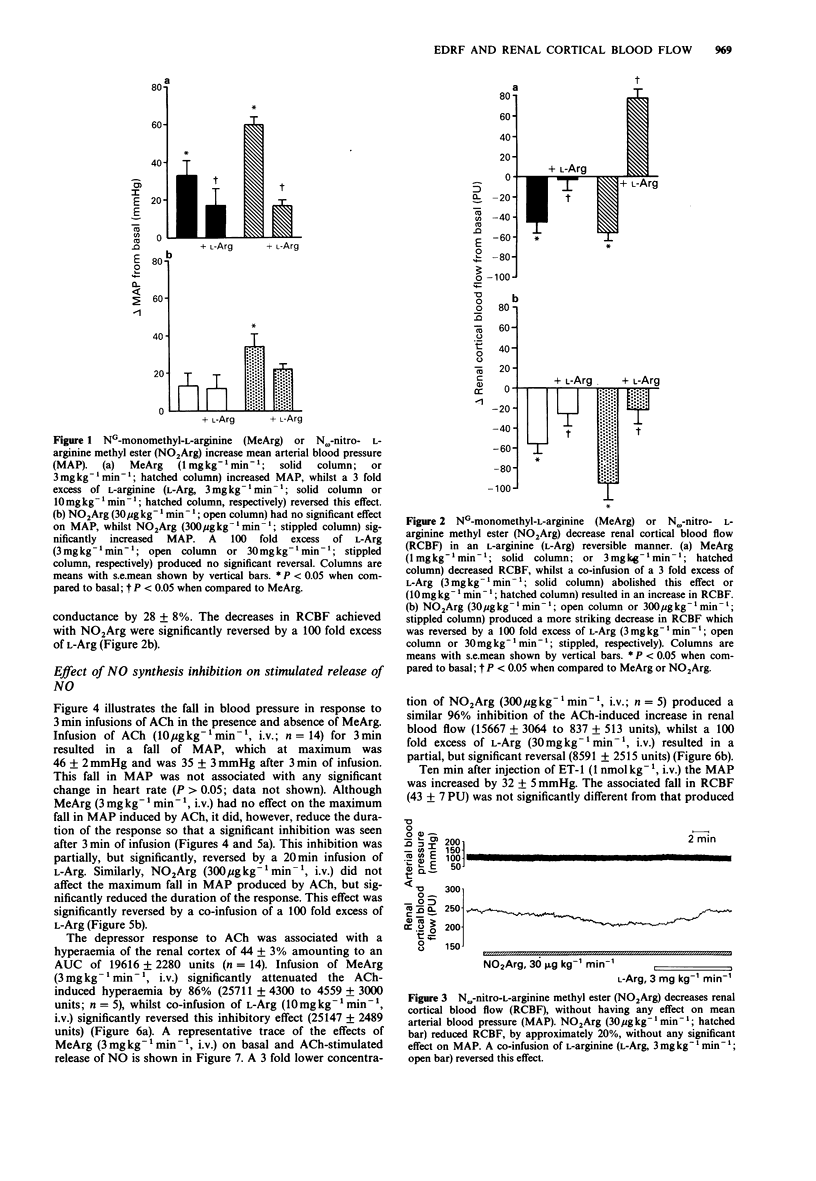
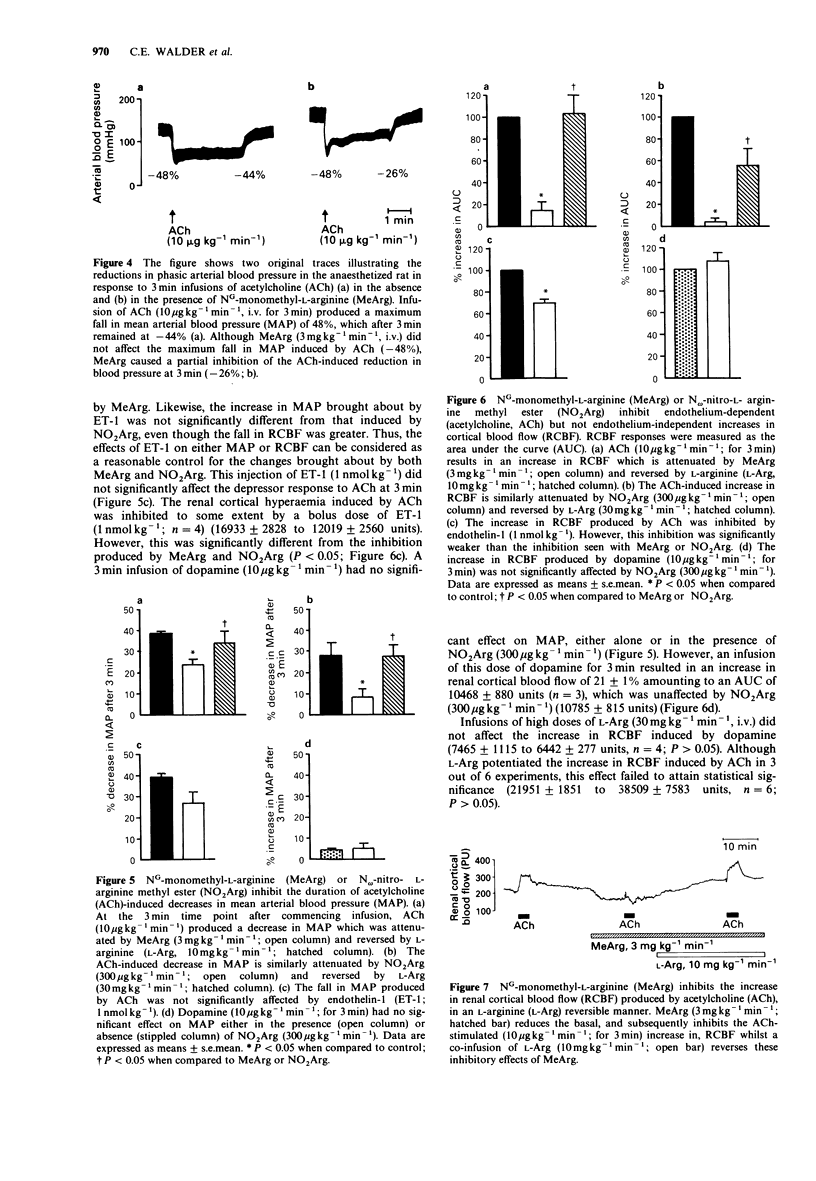
1. In the present study the role of endogenous nitric oxide (NO) was investigated, in the regulation of renal cortical blood flow (RCBF) in vivo in anaesthetized rats under conditions in which prostacyclin involvement had been eliminated. 2. Infusions of the NO synthesis inhibitor NG-monomethyl-L-arginine (MeArg) at 1 or 3 mg kg-1 min-1, i.v., produced significant decreases in RCBF of 29 +/- 7% and 35 +/- 5%, respectively. These effects were reversed by co-infusion of a 3 fold excess of L-arginine (L-Arg). 3. Similarly, intravenous infusion of N omega-nitro-L-arginine methyl ester (NO2Arg) at 30 or 300 micrograms kg-1 min-1 attenuated RCBF by 21 +/- 4% or 53 +/- 4%, respectively, and these effects were reversed by L-Arg (3 or 10 mg kg-1 min-1, i.v.). Most importantly, a low dose of NO2Arg (30 micrograms kg-1 min-1, i.v.), while having no pressor effect, considerably reduced RCBF, indicating that basal release of NO is important for the maintenance of renal cortical blood flow. 4. MeArg (3 mg kg-1 min-1, i.v.) or NO2Arg (300 micrograms kg-1 min-1, i.v.) inhibited endothelium-dependent acetylcholine (ACh, 10 micrograms kg-1 min-1, i.v. for 3 min) increases in RCBF in an L-Arg reversible manner, but did not affect endothelium-independent (dopamine 10 micrograms kg-1 min-1, i.v., for 3 min) increases.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- Aisaka K., Gross S. S., Griffith O. W., Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun. 1989 Apr 28;160(2):881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- Baer P. G., Navar L. G., Guyton A. C. Renal autoregulation, filtration rate, and electrolyte excretion during vasodilatation. Am J Physiol. 1970 Sep;219(3):619–625. doi: 10.1152/ajplegacy.1970.219.3.619. [DOI] [PubMed] [Google Scholar]
- Baylis C., Deen W. M., Myers B. D., Brenner B. M. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976 Apr;230(4):1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R., Moore P. K. Endothelium-derived relaxing factor and the effects of acetylcholine and histamine on resistance blood vessels. Br J Pharmacol. 1988 Nov;95(3):835–843. doi: 10.1111/j.1476-5381.1988.tb11712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj R., Moore P. K. The effect of arginine and nitric oxide on resistance blood vessels of the perfused rat kidney. Br J Pharmacol. 1989 Jul;97(3):739–744. doi: 10.1111/j.1476-5381.1989.tb12011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J. P., Saccaggi A., Lauer A., Ronco C., Belledonne M., Glabman S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 1983 Dec;75(6):943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- Burton G. A., Griffith T. M., Edwards D. H. EDRF-mediated dilatation in the rat isolated perfused kidney: a microangiographic study. Br J Pharmacol. 1989 Dec;98(4):1207–1212. doi: 10.1111/j.1476-5381.1989.tb12666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. A., MacNeil S., de Jonge A., Haylor J. Cyclic GMP release and vasodilatation induced by EDRF and atrial natriuretic factor in the isolated perfused kidney of the rat. Br J Pharmacol. 1990 Feb;99(2):364–368. doi: 10.1111/j.1476-5381.1990.tb14709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J., Shankel S. W. Effects of nonsteroidal anti-inflammatory drugs on prostaglandins and renal function. Am J Med. 1985 Jun;78(6 Pt 1):992–1000. doi: 10.1016/0002-9343(85)90223-2. [DOI] [PubMed] [Google Scholar]
- Cherry P. D., Gillis C. N. Evidence for the role of endothelium-derived relaxing factor in acetylcholine-induced vasodilatation in the intact lung. J Pharmacol Exp Ther. 1987 May;241(2):516–520. [PubMed] [Google Scholar]
- Clive D. M., Stoff J. S. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1984 Mar 1;310(9):563–572. doi: 10.1056/NEJM198403013100905. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1990 Nov;101(3):625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. Endothelium-derived relaxing factor (EDRF) and resistance vessels in an intact vascular bed: a microangiographic study of the rabbit isolated ear. Br J Pharmacol. 1988 Mar;93(3):654–662. doi: 10.1111/j.1476-5381.1988.tb10323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H. Human renal response to meat meal. Am J Physiol. 1986 Apr;250(4 Pt 2):F613–F618. doi: 10.1152/ajprenal.1986.250.4.F613. [DOI] [PubMed] [Google Scholar]
- Ishii K., Chang B., Kerwin J. F., Jr, Huang Z. J., Murad F. N omega-nitro-L-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. Eur J Pharmacol. 1990 Feb 6;176(2):219–223. doi: 10.1016/0014-2999(90)90531-a. [DOI] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Fiksen-Olsen M. J., Raij L., Romero J. C. Effects of NG-monomethyl-L-arginine and L-arginine on acetylcholine renal response. Hypertension. 1990 Jun;15(6 Pt 1):659–663. doi: 10.1161/01.hyp.15.6.659. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. R. Biochemistry and pharmacology of renal arachidonic acid metabolism. Am J Med. 1986 Jan 17;80(1A):3–11. doi: 10.1016/0002-9343(86)90927-7. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Oates J. A., FitzGerald G. A., Branch R. A., Jackson E. K., Knapp H. R., Roberts L. J., 2nd Clinical implications of prostaglandin and thromboxane A2 formation (1). N Engl J Med. 1988 Sep 15;319(11):689–698. doi: 10.1056/NEJM198809153191106. [DOI] [PubMed] [Google Scholar]
- Oates J. A., FitzGerald G. A., Branch R. A., Jackson E. K., Knapp H. R., Roberts L. J., 2nd Clinical implications of prostaglandin and thromboxane A2 formation (2). N Engl J Med. 1988 Sep 22;319(12):761–767. doi: 10.1056/NEJM198809223191206. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Palmore W. P. Glucagon and alanine-induced increases of the canine renal glomerular filtration rate. Q J Exp Physiol. 1983 Jul;68(3):319–327. doi: 10.1113/expphysiol.1983.sp002727. [DOI] [PubMed] [Google Scholar]
- Patrono C., Dunn M. J. The clinical significance of inhibition of renal prostaglandin synthesis. Kidney Int. 1987 Jul;32(1):1–12. doi: 10.1038/ki.1987.164. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva C., Ross B., Benedek G. B. Laser Doppler measurements of blood flow in capillary tubes and retinal arteries. Invest Ophthalmol. 1972 Nov;11(11):936–944. [PubMed] [Google Scholar]
- Smits G. J., Roman R. J., Lombard J. H. Evaluation of laser-Doppler flowmetry as a measure of tissue blood flow. J Appl Physiol (1985) 1986 Aug;61(2):666–672. doi: 10.1152/jappl.1986.61.2.666. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Lappe D. L., Bowen P. D., Chimosky J. E., Holloway G. A., Jr, Keiser H. R., Bowman R. L. Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. Am J Physiol. 1977 Apr;232(4):H441–H448. doi: 10.1152/ajpheart.1977.232.4.H441. [DOI] [PubMed] [Google Scholar]
- Thomas G. R., Thiemermann C., Walder C., Vane J. R. The effects of endothelium-dependent vasodilators on cardiac output and their distribution in the anaesthetized rat: a comparison with sodium nitroprusside. Br J Pharmacol. 1988 Nov;95(3):986–992. doi: 10.1111/j.1476-5381.1988.tb11729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolins J. P., Palmer R. M., Moncada S., Raij L. Role of endothelium-derived relaxing factor in regulation of renal hemodynamic responses. Am J Physiol. 1990 Mar;258(3 Pt 2):H655–H662. doi: 10.1152/ajpheart.1990.258.3.H655. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Walder C. E., Thiemermann C., Vane J. R. Endothelium-derived relaxing factor participates in the increased blood flow in response to pentagastrin in the rat stomach mucosa. Proc Biol Sci. 1990 Sep 22;241(1302):195–200. doi: 10.1098/rspb.1990.0085. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


