Abstract
1. The present study has compared the relative anti-aggregatory effect of various compounds which interfere with thromboxane (Tx) A2-dependent aggregation of human platelets in whole blood in vitro. These included the cyclo-oxygenase inhibitor aspirin, the TxA2 synthase inhibitor dazoxiben, the TxA2 (TP-) receptor blocking drug GR32191 and two compounds, R.68070 ((E)-5-[[[(3-pyridinyl) [3-(trifluoromethyl)phenyl]-methylen] amino]oxy] pentanoic acid) and CV-4151 [E)-7-phenyl-7-(3-pyridyl)-6-heptenoic acid), which possess both TP-receptor blocking and TxA2 synthase inhibitory activities in the same molecule. 2. GR32191, R.68070 and CV-4151 all antagonized aggregation to the TxA2 mimetic U-46619, with pA2 values of approximately 8.2, 5.4 and 4.8 respectively. This effect was specific, platelet aggregation induced by adenosine 5'-diphosphate (ADP) being unaffected by concentrations up to 10, 1000 and 300 microM respectively. In contrast, neither aspirin nor dazoxiben exhibited any measurable TP-receptor blocking activity. 3. The rank order of potency (pIC50) for inhibition of TxA2 formation in serum was R.68070 (7.4) greater than CV-4151 (6.9) greater than dazoxiben (5.7) greater than aspirin (5.3). In addition, all four drugs abolished collagen-induced platelet TxA2 formation. In contrast, GR32191 produced no consistent inhibition of TxA2 formation in either system up to concentrations of 10-30 microM. 4. The specificity of R.68070, CV-4151 and dazoxiben for TxA2 synthase was indicated by their ability to increase serum levels of prostaglandin E2 (PGE2) and PGD2 in parallel with decreases in TxA2 formation. This profile was not observed with aspirin or GR32191.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
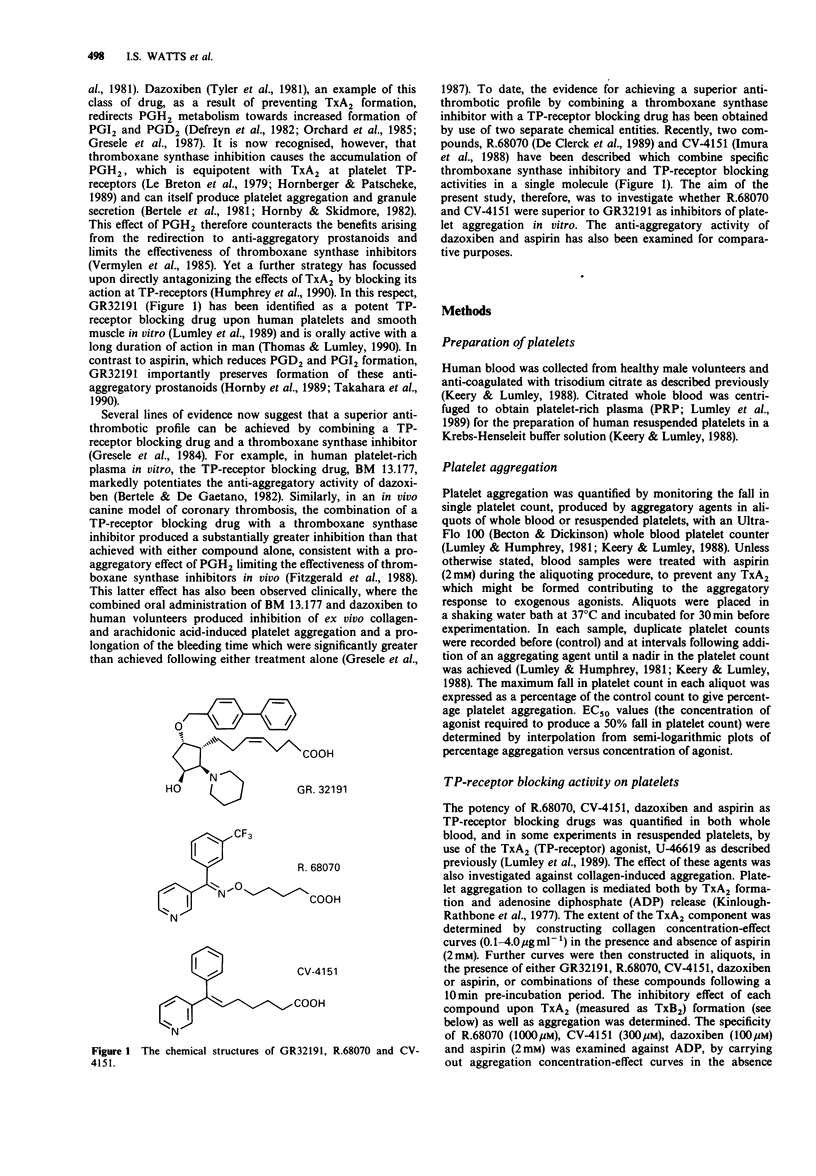

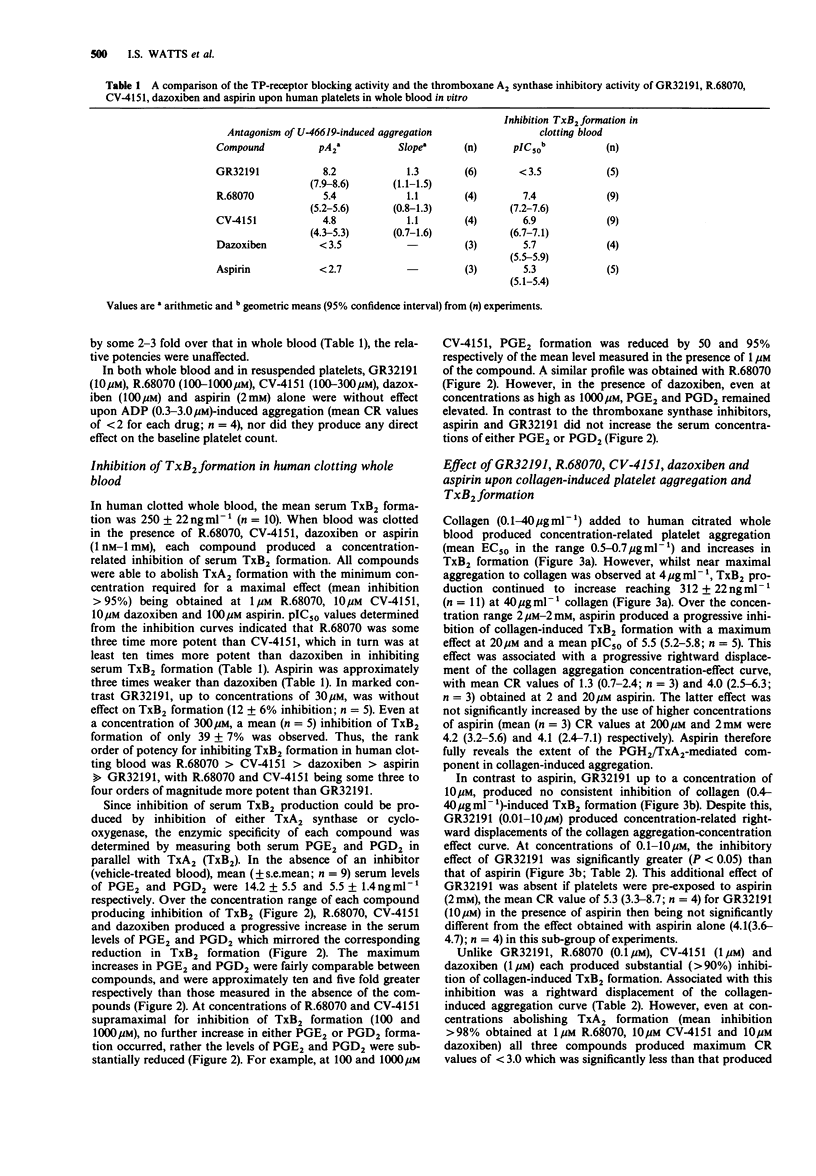
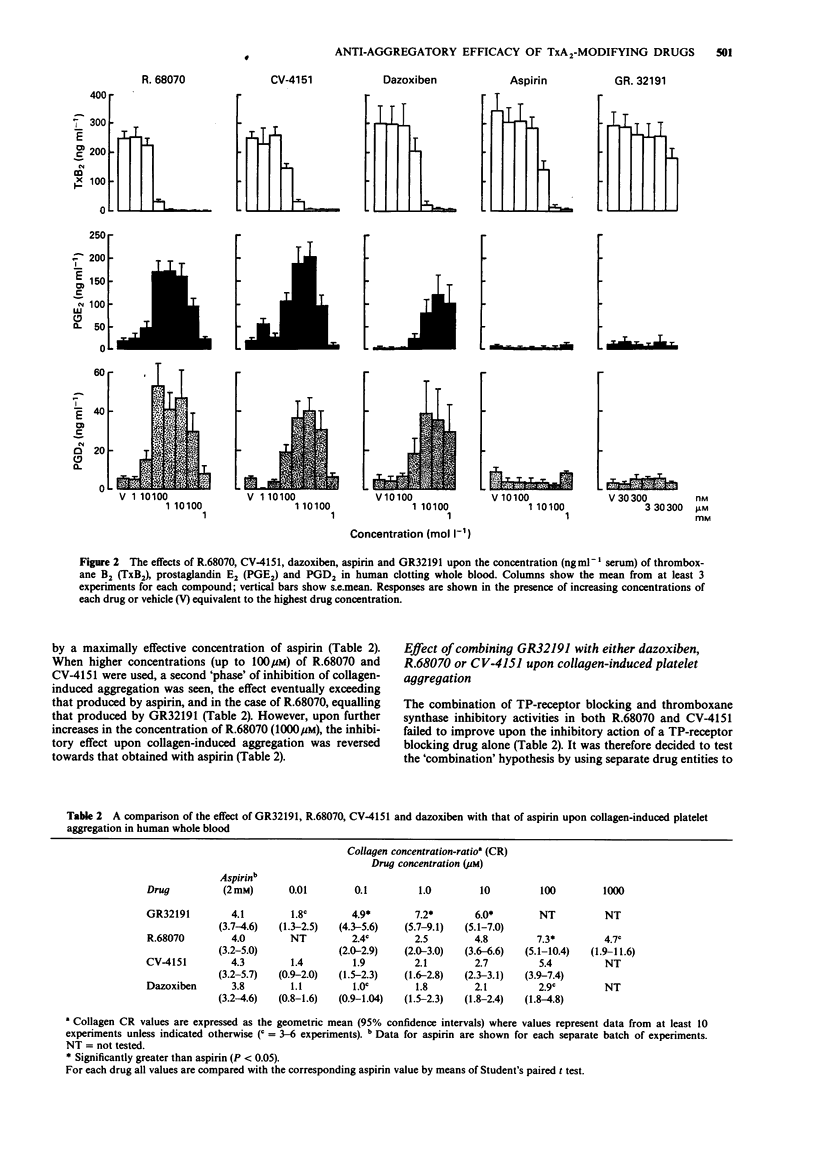
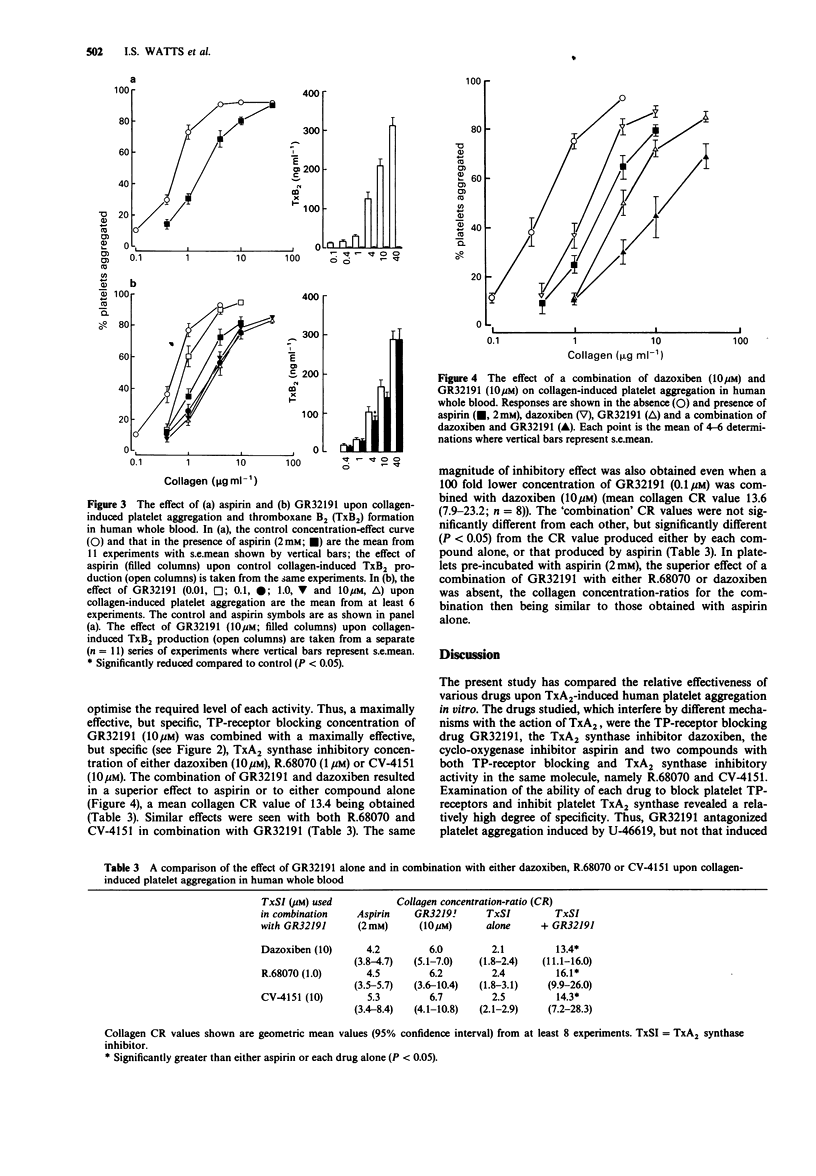



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartele V., Cerletti C., Schiepatti A., di Minno G., de Gaetano G. Inhibition of thromboxane synthetase does not necessarily prevent platelet aggregation. Lancet. 1981 May 9;1(8228):1057–1058. doi: 10.1016/s0140-6736(81)92224-8. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R. Platelet interaction with collagen fibrils in flowing blood. I. Reaction of human platelets with alpha chymotrypsin-digested subendothelium. Thromb Haemost. 1977 Feb 28;37(1):1–16. [PubMed] [Google Scholar]
- Bertele V., De Gaetano G. Potentiation by dazoxiben, a thromboxane synthetase inhibitor, of platelet aggregation inhibitory activity of a thromboxane receptor antagonist and of prostacyclin. Eur J Pharmacol. 1982 Dec 3;85(3-4):331–333. doi: 10.1016/0014-2999(82)90220-5. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Duncombe W. G., Flower R. J., Parsons M. F., Vane J. R. The distribution and metabolism of arachidonic acid in rabbit platelets during aggregation and its modification by drugs. Br J Pharmacol. 1977 Feb;59(2):353–366. doi: 10.1111/j.1476-5381.1977.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catella F., Lawson J. A., Fitzgerald D. J., FitzGerald G. A. Analysis of multiple thromboxane metabolites in plasma and urine. Adv Prostaglandin Thromboxane Leukot Res. 1987;17B:611–614. [PubMed] [Google Scholar]
- De Clerck F., Beetens J., de Chaffoy de Courcelles D., Freyne E., Janssen P. A. R 68 070: thromboxane A2 synthetase inhibition and thromboxane A2/prostaglandin endoperoxide receptor blockade combined in one molecule--I. Biochemical profile in vitro. Thromb Haemost. 1989 Feb 28;61(1):35–42. [PubMed] [Google Scholar]
- Defreyn G., Deckmyn H., Vermylen J. A thromboxane synthetase inhibitor reorients endoperoxide metabolism in whole blood towards prostacyclin and prostaglandin E2. Thromb Res. 1982 Jun 15;26(6):389–400. doi: 10.1016/0049-3848(82)90311-5. [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A., Brash A. R., Oates J. A., Pedersen A. K. Endogenous prostacyclin biosynthesis and platelet function during selective inhibition of thromboxane synthase in man. J Clin Invest. 1983 Oct;72(4):1336–1343. doi: 10.1172/JCI111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. A., Healy C., Daugherty J. Thromboxane A2 biosynthesis in human disease. Fed Proc. 1987 Jan;46(1):154–158. [PubMed] [Google Scholar]
- FitzGerald G. A., Reilly I. A., Pedersen A. K. The biochemical pharmacology of thromboxane synthase inhibition in man. Circulation. 1985 Dec;72(6):1194–1201. doi: 10.1161/01.cir.72.6.1194. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. J., Fragetta J., FitzGerald G. A. Prostaglandin endoperoxides modulate the response to thromboxane synthase inhibition during coronary thrombosis. J Clin Invest. 1988 Nov;82(5):1708–1713. doi: 10.1172/JCI113784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresele P., Arnout J., Deckmyn H., Huybrechts E., Pieters G., Vermylen J. Role of proaggregatory and antiaggregatory prostaglandins in hemostasis. Studies with combined thromboxane synthase inhibition and thromboxane receptor antagonism. J Clin Invest. 1987 Nov;80(5):1435–1445. doi: 10.1172/JCI113223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresele P., Van Houtte E., Arnout J., Deckmyn H., Vermylen J. Thromboxane synthase inhibition combined with thromboxane receptor blockade: a step forward in antithrombotic strategy? Thromb Haemost. 1984 Dec 29;52(3):364–364. [PubMed] [Google Scholar]
- Gréen K., Vesterqvist O. In vivo synthesis of thromboxane and prostacyclin in man in health and disease. Data from GC-MS measurements of major urinary metabolites. Adv Prostaglandin Thromboxane Leukot Res. 1986;16:309–324. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger W. B., Patscheke H. Prostaglandin H2 in human platelet activation: coactivator and substitute for thromboxane A2. Prog Clin Biol Res. 1989;301:315–319. [PubMed] [Google Scholar]
- Hornby E. J., Foster M. R., McCabe P. J., Stratton L. E. The inhibitory effect of GR32191, a thromboxane receptor blocking drug, on human platelet aggregation, adhesion and secretion. Thromb Haemost. 1989 Jun 30;61(3):429–436. [PubMed] [Google Scholar]
- Hornby E. J., Skidmore I. F. Evidence that prostaglandin endoperoxides can induce platelet aggregation in the absence of thromboxane A2 production. Biochem Pharmacol. 1982 Mar 15;31(6):1158–1160. doi: 10.1016/0006-2952(82)90359-8. [DOI] [PubMed] [Google Scholar]
- Imura Y., Terashita Z., Shibouta Y., Nishikawa K. The thromboxane A2/prostaglandin endoperoxide receptor antagonist activity of CV-4151, a thromboxane A2 synthetase inhibitor. Eur J Pharmacol. 1988 Mar 15;147(3):359–365. doi: 10.1016/0014-2999(88)90169-0. [DOI] [PubMed] [Google Scholar]
- Keery R. J., Lumley P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br J Pharmacol. 1988 Jul;94(3):745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy I., Coleman R. A., Humphrey P. P., Levy G. P., Lumley P. Studies on the characterisation of prostanoid receptors: a proposed classification. Prostaglandins. 1982 Nov;24(5):667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Packham M. A., Reimers H. J., Cazenave J. P., Mustard J. F. Mechanisms of platelet shape change, aggregation, and release induced by collagen, thrombin, or A23,187. J Lab Clin Med. 1977 Oct;90(4):707–719. [PubMed] [Google Scholar]
- Le Breton G. C., Venton D. L., Enke S. E., Halushka P. V. 13-Azaprostanoic acid: a specific antagonist of the human blood platelet thromboxane/endoperoxide receptor. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4097–4101. doi: 10.1073/pnas.76.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley P., Humphrey P. P. A method for quantitating platelet aggregation and analyzing drug-receptor interactions on platelets in whole blood in vitro. J Pharmacol Methods. 1981 Sep;6(2):153–166. doi: 10.1016/0160-5402(81)90038-3. [DOI] [PubMed] [Google Scholar]
- Lumley P., White B. P., Humphrey P. P. GR32191, a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle in vitro. Br J Pharmacol. 1989 Jul;97(3):783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievelstein P. F., de Groot P. G. Interaction of blood platelets with the vessel wall. Haemostasis. 1988;18(4-6):342–359. doi: 10.1159/000215816. [DOI] [PubMed] [Google Scholar]
- Orchard M. A., Waddell K. A., Lewis P. J., Blair I. A. Thromboxane synthase inhibition causes re-direction of prostaglandin endoperoxides to prostaglandin D2 during collagen stimulated aggregation of human platelet rich plasma. Thromb Res. 1985 Sep 15;39(6):701–710. doi: 10.1016/0049-3848(85)90254-3. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Takahara K., Murray R., FitzGerald G. A., Fitzgerald D. J. The response to thromboxane A2 analogues in human platelets. Discrimination of two binding sites linked to distinct effector systems. J Biol Chem. 1990 Apr 25;265(12):6836–6844. [PubMed] [Google Scholar]
- Terashita Z., Imura Y., Tanabe M., Kawazoe K., Nishikawa K., Kato K., Terao S. CV-4151--a potent, selective thromboxane A2 synthetase inhibitor. Thromb Res. 1986 Jan 15;41(2):223–237. doi: 10.1016/0049-3848(86)90231-8. [DOI] [PubMed] [Google Scholar]
- Thomas M., Lumley P. Preliminary assessment of a novel thromboxane A2 receptor-blocking drug, GR32191, in healthy subjects. Circulation. 1990 Jan;81(1 Suppl):I53–I60. [PubMed] [Google Scholar]
- Tyler H. M., Saxton C. A., Parry M. J. Administration to man of UK-37,248-01, a selective inhibitor of thromboxane synthetase. Lancet. 1981 Mar 21;1(8221):629–632. doi: 10.1016/s0140-6736(81)91551-8. [DOI] [PubMed] [Google Scholar]
- Vermylen J., Defreyn G., Carreras L. O., Machin S. J., Van Schaeren J., Verstraete M. Thromboxane synthetase inhibition as antithrombotic strategy. Lancet. 1981 May 16;1(8229):1073–1075. doi: 10.1016/s0140-6736(81)92241-8. [DOI] [PubMed] [Google Scholar]


