Abstract
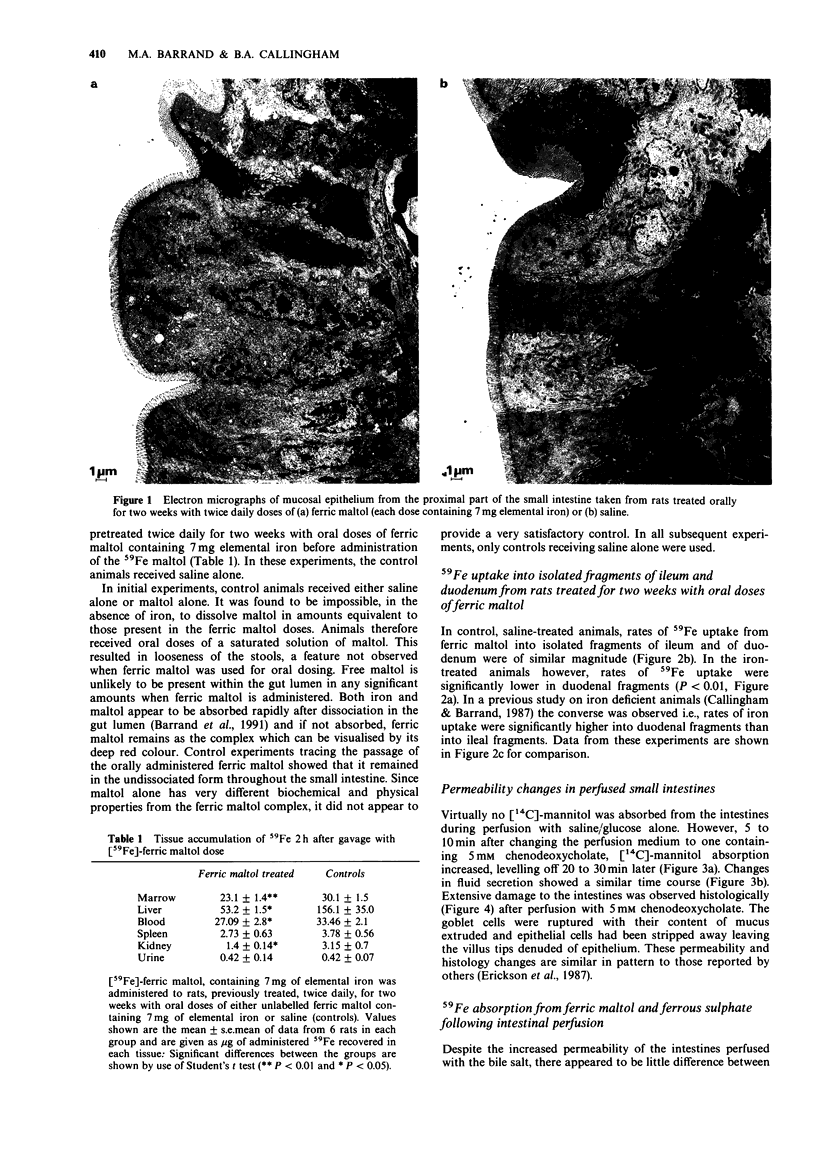
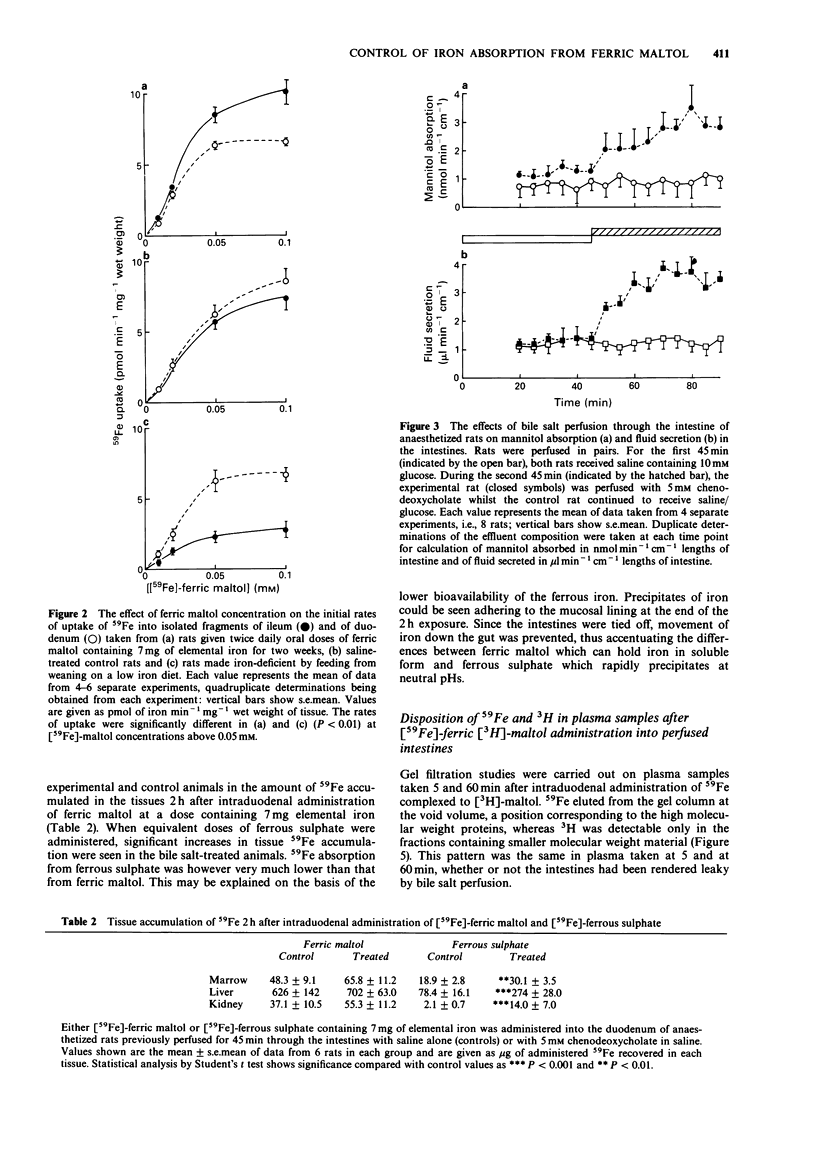
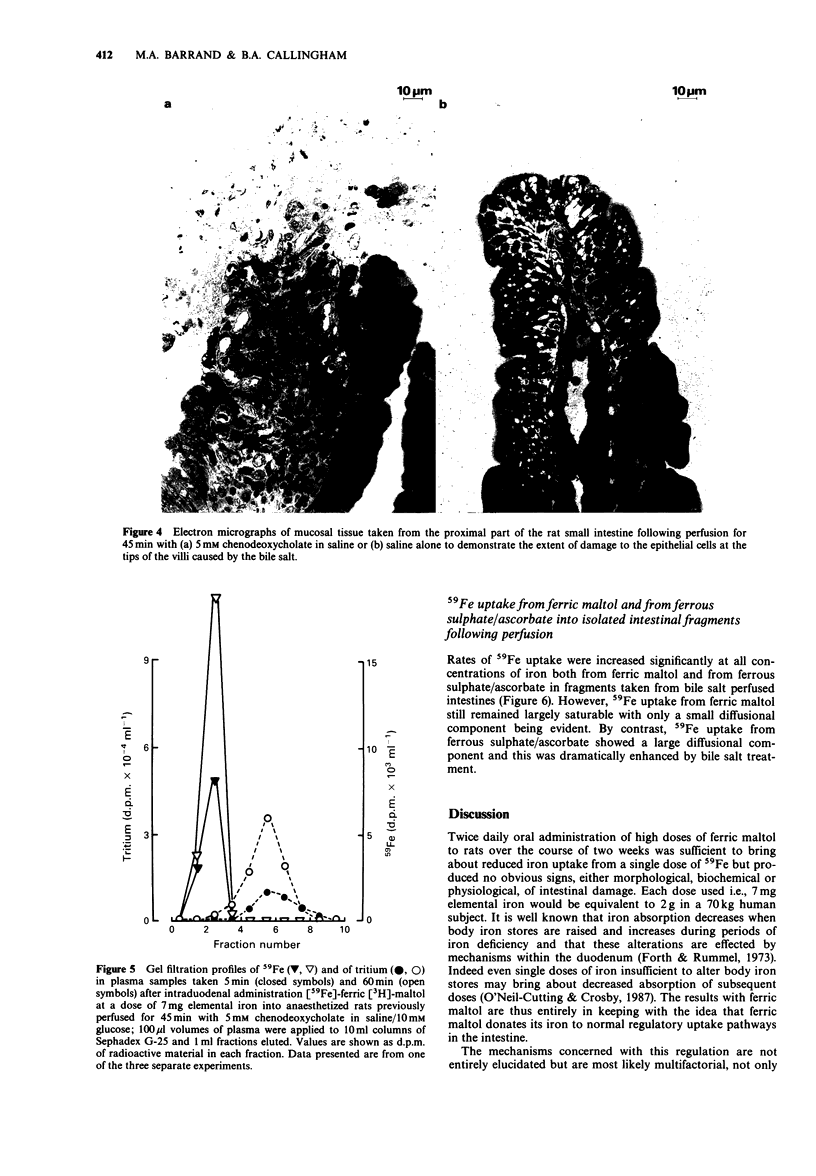
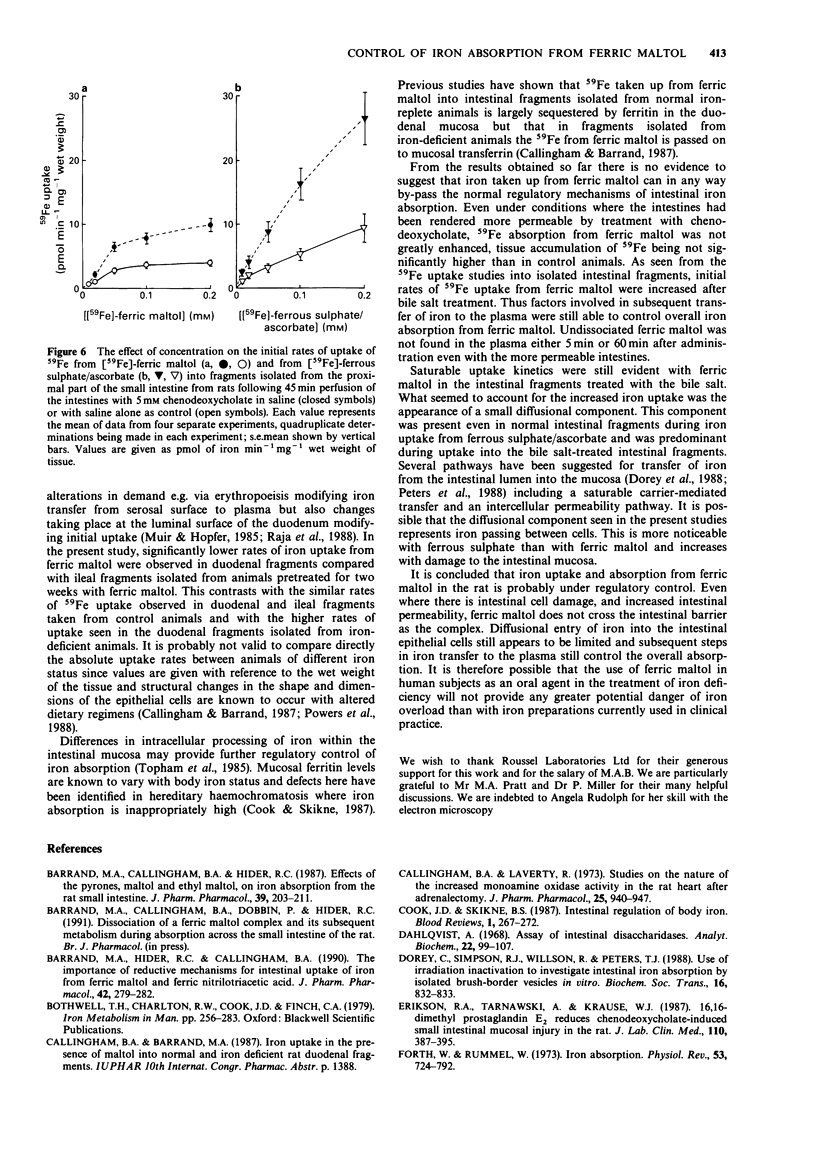
1. 59Fe absorption from the novel iron compound, ferric maltol, was studied in rats pretreated twice daily for two weeks with non-radioactive ferric maltol in oral doses containing 7 mg elemental iron. Tissue accumulation of 59Fe 2 h after administration of radioactive ferric maltol into the stomach was significantly lower in iron pretreated animals than in saline-treated controls. 2. 59Fe uptake from ferric maltol into isolated fragments of ileum and of duodenum was of similar magnitude in control animals but in iron-treated animals, duodenal uptake was significantly lower than that of the ileum. 3. Absorption of 59Fe was also investigated in anaesthetized rats after intestinal perfusion with saline (controls) or with 5 mM chenodeoxycholate to render the intestines more permeable. 4. Changes in permeability of the small intestine were monitored by estimating the amount of [14C]-mannitol absorbed and fluid secreted with reference to the non-absorbable [3H]-inulin in the perfusate effluents. 5. Despite the increased permeability of the intestines after bile salt treatment, there was little difference from control in the tissue accumulation of 59Fe from ferric maltol 2 h after intraduodenal administration. However 59Fe absorption from ferrous sulphate was significantly increased after bile salt treatment. 6. Gel filtration profiles of plasma made 5 and 60 min after intraduodenal administration of [59Fe]-ferric [3H]-maltol demonstrated that metal and ligand do not enter the circulation as the complex even when intestinal permeability is increased. 7. Uptake of 59Fe was investigated in isolated fragments of rat small intestine after saline or bile salt perfusion. Although 59Fe uptake from ferric maltol was somewhat greater in the bile salt-treated intestinal fragments, saturable kinetics were still observed.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrand M. A., Callingham B. A., Hider R. C. Effects of the pyrones, maltol and ethyl maltol, on iron absorption from the rat small intestine. J Pharm Pharmacol. 1987 Mar;39(3):203–211. doi: 10.1111/j.2042-7158.1987.tb06249.x. [DOI] [PubMed] [Google Scholar]
- Barrand M. A., Hider R. C., Callingham B. A. The importance of reductive mechanisms for intestinal uptake of iron from ferric maltol and ferric nitrilotriacetic acid (NTA). J Pharm Pharmacol. 1990 Apr;42(4):279–282. doi: 10.1111/j.2042-7158.1990.tb05408.x. [DOI] [PubMed] [Google Scholar]
- Callingham B. A., Laverty R. Studies on the nature of the increased monoamine oxidase activity in the rat heart after adrenalectomy. J Pharm Pharmacol. 1973 Dec;25(12):940–947. doi: 10.1111/j.2042-7158.1973.tb09983.x. [DOI] [PubMed] [Google Scholar]
- Cook J. D., Skikne B. S. Intestinal regulation of body iron. Blood Rev. 1987 Dec;1(4):267–272. doi: 10.1016/0268-960x(87)90028-2. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Erickson R. A., Tarnawski A., Krause W. J. 16,16-Dimethyl prostaglandin E2 reduced chenodeoxycholate-induced small intestinal mucosal injury in the rat. J Lab Clin Med. 1987 Oct;110(4):387–395. [PubMed] [Google Scholar]
- Forth W., Rummel W. Iron absorption. Physiol Rev. 1973 Jul;53(3):724–792. doi: 10.1152/physrev.1973.53.3.724. [DOI] [PubMed] [Google Scholar]
- Levey J. A., Barrand M. A., Callingham B. A., Hider R. C. Characteristics of iron(III) uptake by isolated fragments of rat small intestine in the presence of the hydroxypyrones, maltol and ethyl maltol. Biochem Pharmacol. 1988 May 15;37(10):2051–2057. doi: 10.1016/0006-2952(88)90556-4. [DOI] [PubMed] [Google Scholar]
- McLaren G. D., Nathanson M. H., Jacobs A., Trevett D., Thomson W. Control of iron absorption in hemochromatosis. Mucosal iron kinetics in vivo. Ann N Y Acad Sci. 1988;526:185–198. doi: 10.1111/j.1749-6632.1988.tb55505.x. [DOI] [PubMed] [Google Scholar]
- Muir A., Hopfer U. Regional specificity of iron uptake by small intestinal brush-border membranes from normal and iron-deficient mice. Am J Physiol. 1985 Mar;248(3 Pt 1):G376–G379. doi: 10.1152/ajpgi.1985.248.3.G376. [DOI] [PubMed] [Google Scholar]
- O'Neil-Cutting M. A., Crosby W. H. Blocking of iron absorption by a preliminary oral dose of iron. Arch Intern Med. 1987 Mar;147(3):489–491. [PubMed] [Google Scholar]
- Peters T. J., Raja K. B., Simpson R. J., Snape S. Mechanisms and regulation of intestinal iron absorption. Ann N Y Acad Sci. 1988;526:141–147. doi: 10.1111/j.1749-6632.1988.tb55500.x. [DOI] [PubMed] [Google Scholar]
- Powers H. J., Wright A. J., Fairweather-Tait S. J. The effect of riboflavin deficiency in rats on the absorption and distribution of iron. Br J Nutr. 1988 May;59(3):381–387. doi: 10.1079/bjn19880047. [DOI] [PubMed] [Google Scholar]
- Raja K. B., Simpson R. J., Pippard M. J., Peters T. J. In vivo studies on the relationship between intestinal iron (Fe3+) absorption, hypoxia and erythropoiesis in the mouse. Br J Haematol. 1988 Mar;68(3):373–378. doi: 10.1111/j.1365-2141.1988.tb04217.x. [DOI] [PubMed] [Google Scholar]
- Topham R. W., Joslin S. A., Prince J. S., Jr The effect of short-term exposure to low-iron diets on the mucosal processing of ionic iron. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1092–1097. doi: 10.1016/0006-291x(85)91248-3. [DOI] [PubMed] [Google Scholar]




