Abstract
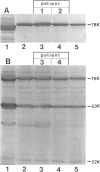
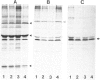
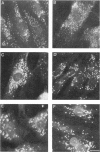
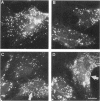
We describe four infants with a novel subtype of an isolated deficiency of one of the peroxisomal β-oxidation enzymes with detectable enzyme protein. The patients showed characteristic clinical and biochemical abnormalities, including hypotonia, psychomotor retardation, hepatomegaly, typical facial appearance, accumulation of very-long-chain fatty acids, and decreased lignoceric acid oxidation. However, β-oxidation enzyme proteins were detected by immunoblot analyses, and large peroxisomes were identified by immunofluorescence staining. In order to identify the underlying defect in these patients, complementation analysis was introduced using fibroblasts from these patients and patients with an established deficiency of either acyl-CoA oxidase or bifunctional enzyme, as identified by immunoblotting. In the complementing combinations, fused cells showed increased lignoceric acid oxidation, resistance against 1-pyrene dodecanoic acid/UV selection, and normalization of the size and the distribution of peroxisomes. The results indicate that two patients with a more severe clinical course were suffering from bifunctional enzyme deficiency and that the other two infants, who were siblings and had a less severe clinical presentation, were the first patients with acyl-CoA oxidase deficiency with detectable enzyme protein.
Full text
PDF
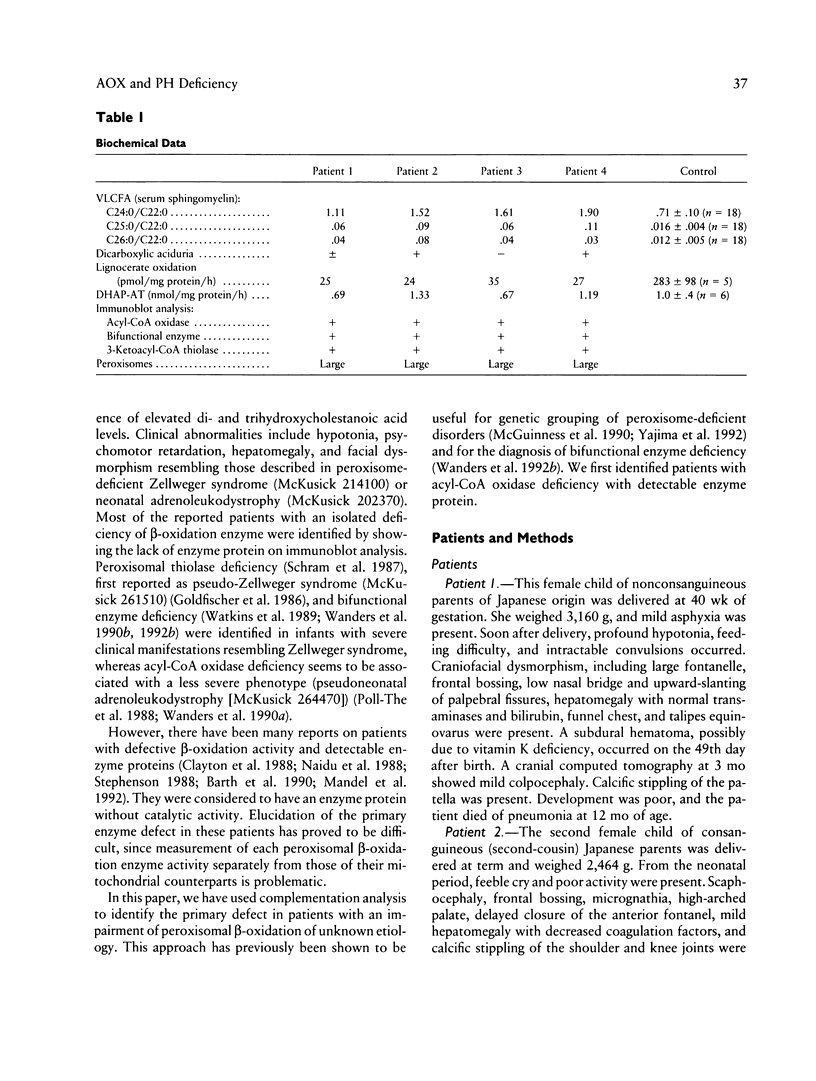
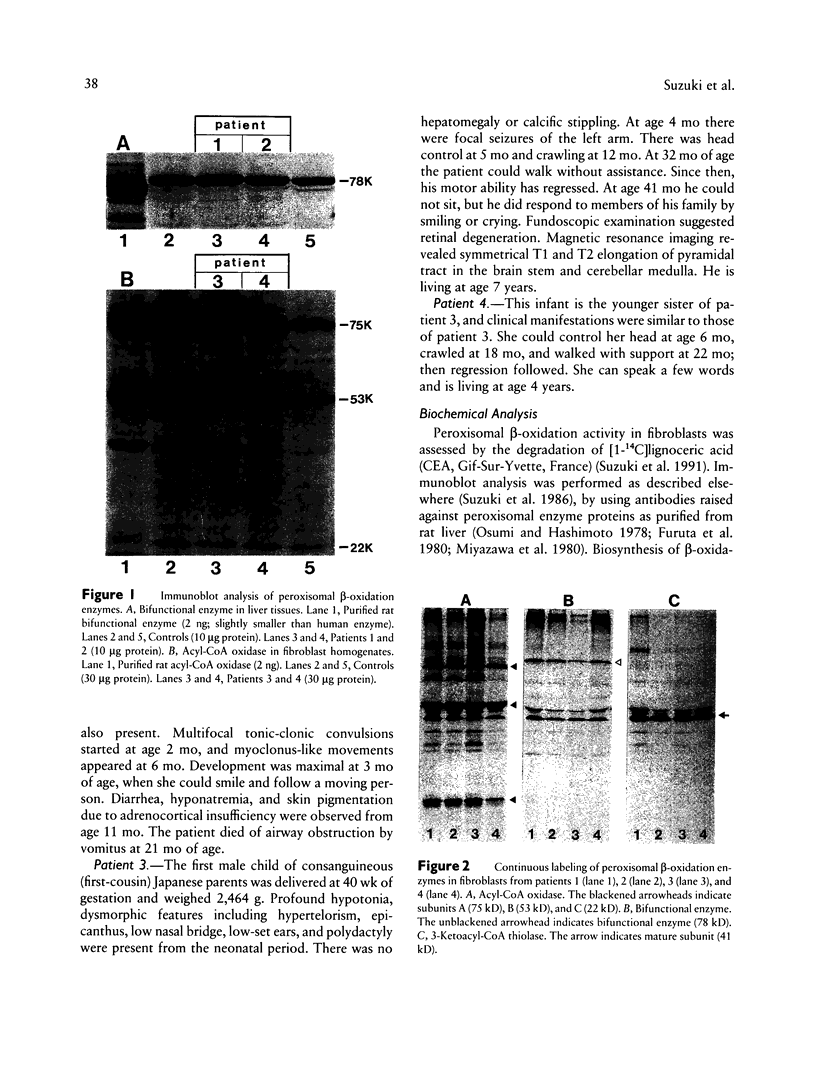
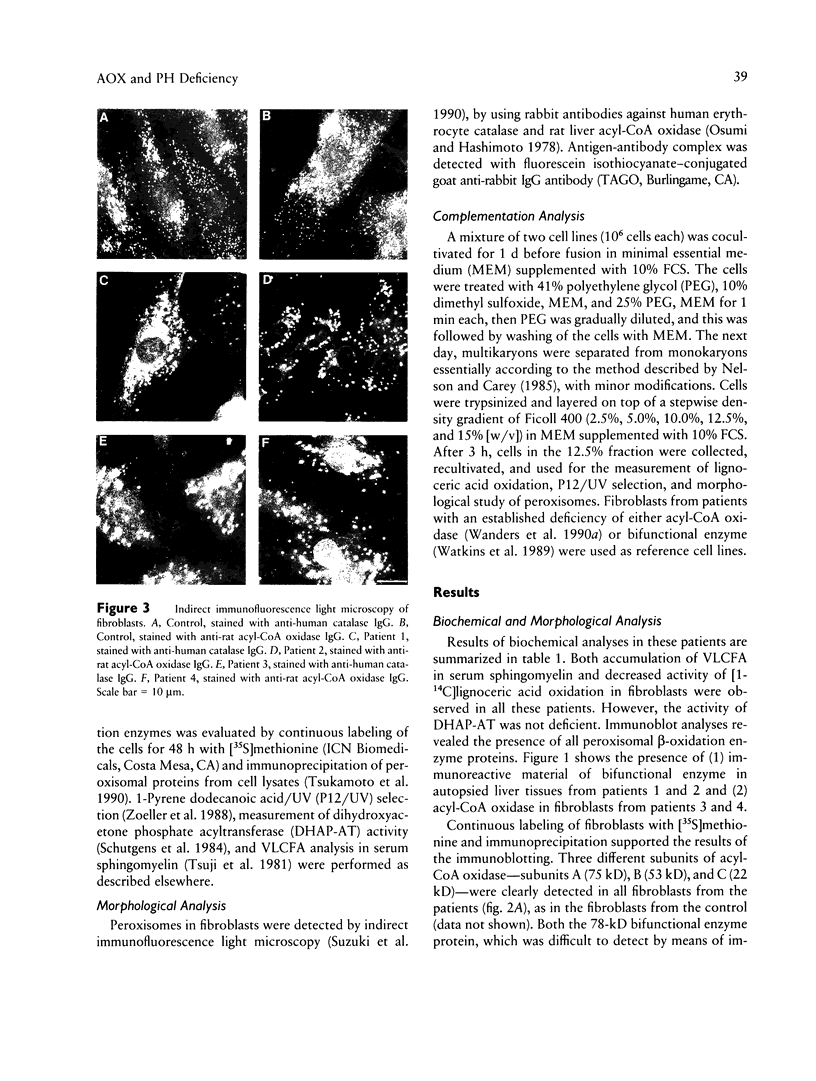
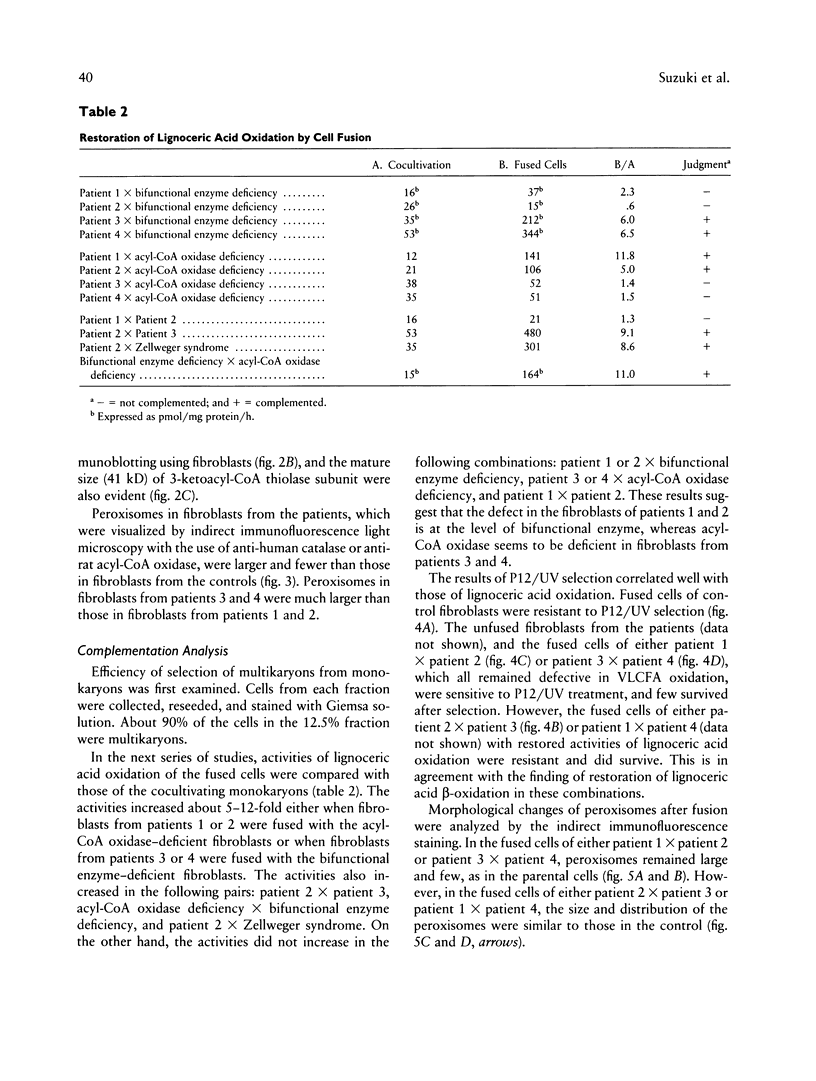
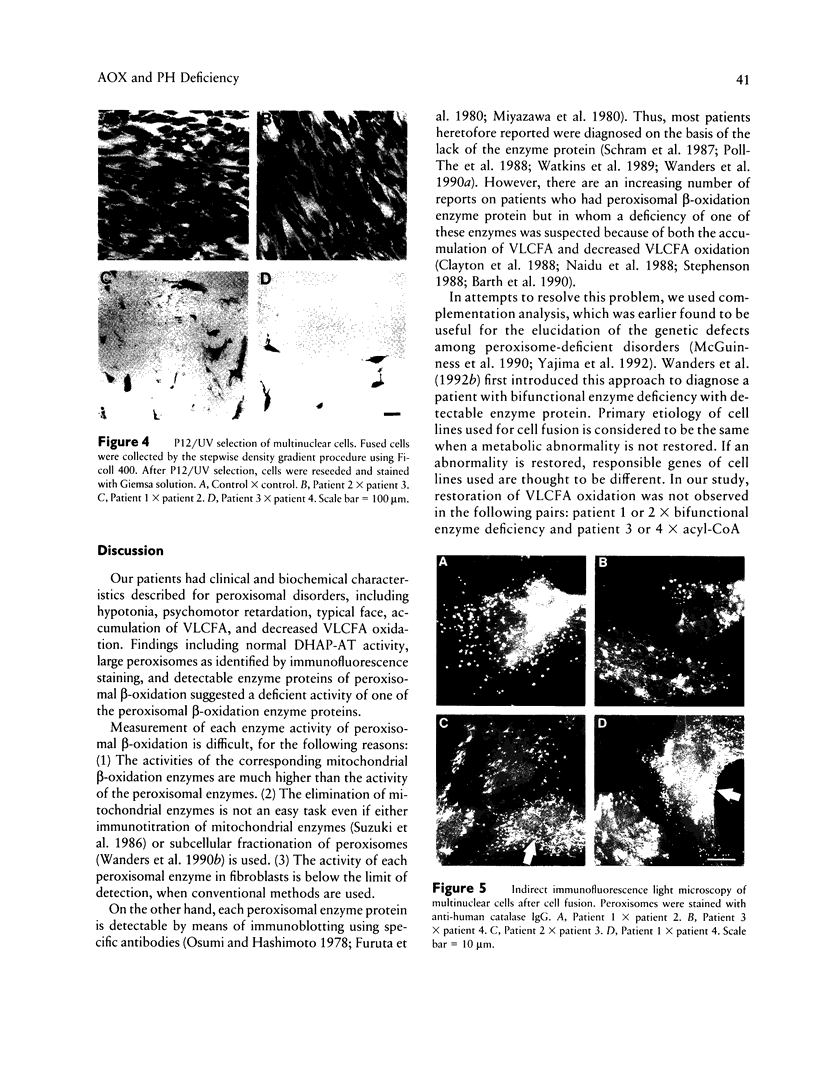


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth P. G., Wanders R. J., Schutgens R. B., Bleeker-Wagemakers E. M., van Heemstra D. Peroxisomal beta-oxidation defect with detectable peroxisomes: a case with neonatal onset and progressive course. Eur J Pediatr. 1990 Jul;149(10):722–726. doi: 10.1007/BF01959531. [DOI] [PubMed] [Google Scholar]
- Casteels M., Schepers L., Van Eldere J., Eyssen H. J., Mannaerts G. P. Inhibition of 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoic acid oxidation and of bile acid secretion in rat liver by fatty acids. J Biol Chem. 1988 Apr 5;263(10):4654–4661. [PubMed] [Google Scholar]
- Clayton P. T., Lake B. D., Hjelm M., Stephenson J. B., Besley G. T., Wanders R. J., Schram A. W., Tager J. M., Schutgens R. B., Lawson A. M. Bile acid analyses in "pseudo-Zellweger" syndrome; clues to the defect in peroxisomal beta-oxidation. J Inherit Metab Dis. 1988;11 (Suppl 2):165–168. doi: 10.1007/BF01804226. [DOI] [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Osumi T., Hashimoto T., Ui N. Properties of mitochondria and peroxisomal enoyl-CoA hydratases from rat liver. J Biochem. 1980 Oct;88(4):1059–1070. doi: 10.1093/oxfordjournals.jbchem.a133057. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Collins J., Rapin I., Neumann P., Neglia W., Spiro A. J., Ishii T., Roels F., Vamecq J., Van Hoof F. Pseudo-Zellweger syndrome: deficiencies in several peroxisomal oxidative activities. J Pediatr. 1986 Jan;108(1):25–32. doi: 10.1016/s0022-3476(86)80764-8. [DOI] [PubMed] [Google Scholar]
- Hoefler G., Paschke E., Hoefler S., Moser A. B., Moser H. W. Photosensitized killing of cultured fibroblasts from patients with peroxisomal disorders due to pyrene fatty acid-mediated ultraviolet damage. J Clin Invest. 1991 Dec;88(6):1873–1879. doi: 10.1172/JCI115509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase B. F., Björkhem I., Hågå P., Pedersen J. I. Defective peroxisomal cleavage of the C27-steroid side chain in the cerebro-hepato-renal syndrome of Zellweger. J Clin Invest. 1985 Feb;75(2):427–435. doi: 10.1172/JCI111717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel H., Berant M., Aizin A., Gershony R., Hemmli S., Schutgens R. B., Wanders R. J. Zellweger-like phenotype in two siblings: a defect in peroxisomal beta-oxidation with elevated very long-chain fatty acids but normal bile acids. J Inherit Metab Dis. 1992;15(3):381–384. doi: 10.1007/BF02435982. [DOI] [PubMed] [Google Scholar]
- McGuinness M. C., Moser A. B., Moser H. W., Watkins P. A. Peroxisomal disorders: complementation analysis using beta-oxidation of very long chain fatty acids. Biochem Biophys Res Commun. 1990 Oct 15;172(1):364–369. doi: 10.1016/s0006-291x(05)80218-9. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T. The presence of a new 3-oxoacyl-CoA thiolase in rat liver peroxisomes. Eur J Biochem. 1980 Feb;103(3):589–596. doi: 10.1111/j.1432-1033.1980.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Naidu S., Hoefler G., Watkins P. A., Chen W. W., Moser A. B., Hoefler S., Rance N. E., Powers J. M., Beard M., Green W. R. Neonatal seizures and retardation in a girl with biochemical features of X-linked adrenoleukodystrophy: a possible new peroxisomal disease entity. Neurology. 1988 Jul;38(7):1100–1107. doi: 10.1212/wnl.38.7.1100. [DOI] [PubMed] [Google Scholar]
- Nelson P. V., Carey W. F. A method for enrichment of hybrid somatic cells: complementation studies in certain lysosomal enzymopathies. J Inherit Metab Dis. 1985;8(3):95–99. doi: 10.1007/BF01819286. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Acyl-CoA oxidase of rat liver: a new enzyme for fatty acid oxidation. Biochem Biophys Res Commun. 1978 Jul 28;83(2):479–485. doi: 10.1016/0006-291x(78)91015-x. [DOI] [PubMed] [Google Scholar]
- Palosaari P. M., Hiltunen J. K. Peroxisomal bifunctional protein from rat liver is a trifunctional enzyme possessing 2-enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and delta 3, delta 2-enoyl-CoA isomerase activities. J Biol Chem. 1990 Feb 15;265(5):2446–2449. [PubMed] [Google Scholar]
- Poll-The B. T., Roels F., Ogier H., Scotto J., Vamecq J., Schutgens R. B., Wanders R. J., van Roermund C. W., van Wijland M. J., Schram A. W. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). Am J Hum Genet. 1988 Mar;42(3):422–434. [PMC free article] [PubMed] [Google Scholar]
- Schram A. W., Goldfischer S., van Roermund C. W., Brouwer-Kelder E. M., Collins J., Hashimoto T., Heymans H. S., van den Bosch H., Schutgens R. B., Tager J. M. Human peroxisomal 3-oxoacyl-coenzyme A thiolase deficiency. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2494–2496. doi: 10.1073/pnas.84.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens R. B., Romeyn G. J., Wanders R. J., van den Bosch H., Schrakamp G., Heymans H. S. Deficiency of acyl-CoA: dihydroxyacetone phosphate acyltransferase in patients with Zellweger (cerebro-hepato-renal) syndrome. Biochem Biophys Res Commun. 1984 Apr 16;120(1):179–184. doi: 10.1016/0006-291x(84)91430-x. [DOI] [PubMed] [Google Scholar]
- Singh I., Moser A. E., Goldfischer S., Moser H. W. Lignoceric acid is oxidized in the peroxisome: implications for the Zellweger cerebro-hepato-renal syndrome and adrenoleukodystrophy. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4203–4207. doi: 10.1073/pnas.81.13.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. B. Inherited peroxisomal disorders involving the nervous system. Arch Dis Child. 1988 Jul;63(7):767–770. doi: 10.1136/adc.63.7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Orii T., Mori M., Tatibana M., Hashimoto T. Deficient activities and proteins of peroxisomal beta-oxidation enzymes in infants with Zellweger syndrome. Clin Chim Acta. 1986 Apr 30;156(2):191–196. doi: 10.1016/0009-8981(86)90152-x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Shimozawa N., Yajima S., Yamaguchi S., Orii T., Hashimoto T. Effects of sodium 2-[5-(4-chlorophenyl)pentyl]-oxirane-2-carboxylate (POCA) on fatty acid oxidation in fibroblasts from patients with peroxisomal diseases. Biochem Pharmacol. 1991 Feb 1;41(3):453–456. doi: 10.1016/0006-2952(91)90544-f. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yamaguchi S., Orii T., Tsuneoka M., Tashiro Y. Nonspecific lipid transfer protein (sterol carrier protein-2) defective in patients with deficient peroxisomes. Cell Struct Funct. 1990 Oct;15(5):301–308. doi: 10.1247/csf.15.301. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Suzuki M., Ariga T., Sekine M., Kuriyama M., Miyatake T. Abnormality of long-chain fatty acids in erythrocyte membrane sphingomyelin from patients with adrenoleukodystrophy. J Neurochem. 1981 Mar;36(3):1046–1049. doi: 10.1111/j.1471-4159.1981.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Yokota S., Fujiki Y. Isolation and characterization of Chinese hamster ovary cell mutants defective in assembly of peroxisomes. J Cell Biol. 1990 Mar;110(3):651–660. doi: 10.1083/jcb.110.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Vanhove G., Assselberghs S., Eyssen H. J., Mannaerts G. P. Substrate specificities of rat liver peroxisomal acyl-CoA oxidases: palmitoyl-CoA oxidase (inducible acyl-CoA oxidase), pristanoyl-CoA oxidase (non-inducible acyl-CoA oxidase), and trihydroxycoprostanoyl-CoA oxidase. J Biol Chem. 1992 Oct 5;267(28):20065–20074. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Vanhove G., Vanhoutte F., Dacremont G., Parmentier G., Eyssen H. J., Mannaerts G. P. Identification and purification of a peroxisomal branched chain fatty acyl-CoA oxidase. J Biol Chem. 1991 Dec 25;266(36):24676–24683. [PubMed] [Google Scholar]
- Wanders R. J., Denis S., Jakobs C., ten Brink H. J. Identification of pristanoyl-CoA oxidase as a distinct, clofibrate non-inducible enzyme in rat liver peroxisomes. Biochim Biophys Acta. 1992 Mar 4;1124(2):199–202. doi: 10.1016/0005-2760(92)90099-h. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Schelen A., Feller N., Schutgens R. B., Stellaard F., Jakobs C., Mitulla B., Seidlitz G. First prenatal diagnosis of acyl-CoA oxidase deficiency. J Inherit Metab Dis. 1990;13(3):371–374. doi: 10.1007/BF01799398. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., Brul S., Schutgens R. B., Tager J. M. Bifunctional enzyme deficiency: identification of a new type of peroxisomal disorder in a patient with an impairment in peroxisomal beta-oxidation of unknown aetiology by means of complementation analysis. J Inherit Metab Dis. 1992;15(3):385–388. doi: 10.1007/BF02435983. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., Schelen A., Schutgens R. B., Tager J. M., Stephenson J. B., Clayton P. T. A bifunctional protein with deficient enzymic activity: identification of a new peroxisomal disorder using novel methods to measure the peroxisomal beta-oxidation enzyme activities. J Inherit Metab Dis. 1990;13(3):375–379. doi: 10.1007/BF01799399. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Chen W. W., Harris C. J., Hoefler G., Hoefler S., Blake D. C., Jr, Balfe A., Kelley R. I., Moser A. B., Beard M. E. Peroxisomal bifunctional enzyme deficiency. J Clin Invest. 1989 Mar;83(3):771–777. doi: 10.1172/JCI113956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima S., Suzuki Y., Shimozawa N., Yamaguchi S., Orii T., Fujiki Y., Osumi T., Hashimoto T., Moser H. W. Complementation study of peroxisome-deficient disorders by immunofluorescence staining and characterization of fused cells. Hum Genet. 1992 Mar;88(5):491–499. doi: 10.1007/BF00219334. [DOI] [PubMed] [Google Scholar]
- Zoeller R. A., Morand O. H., Raetz C. R. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988 Aug 15;263(23):11590–11596. [PubMed] [Google Scholar]