Abstract
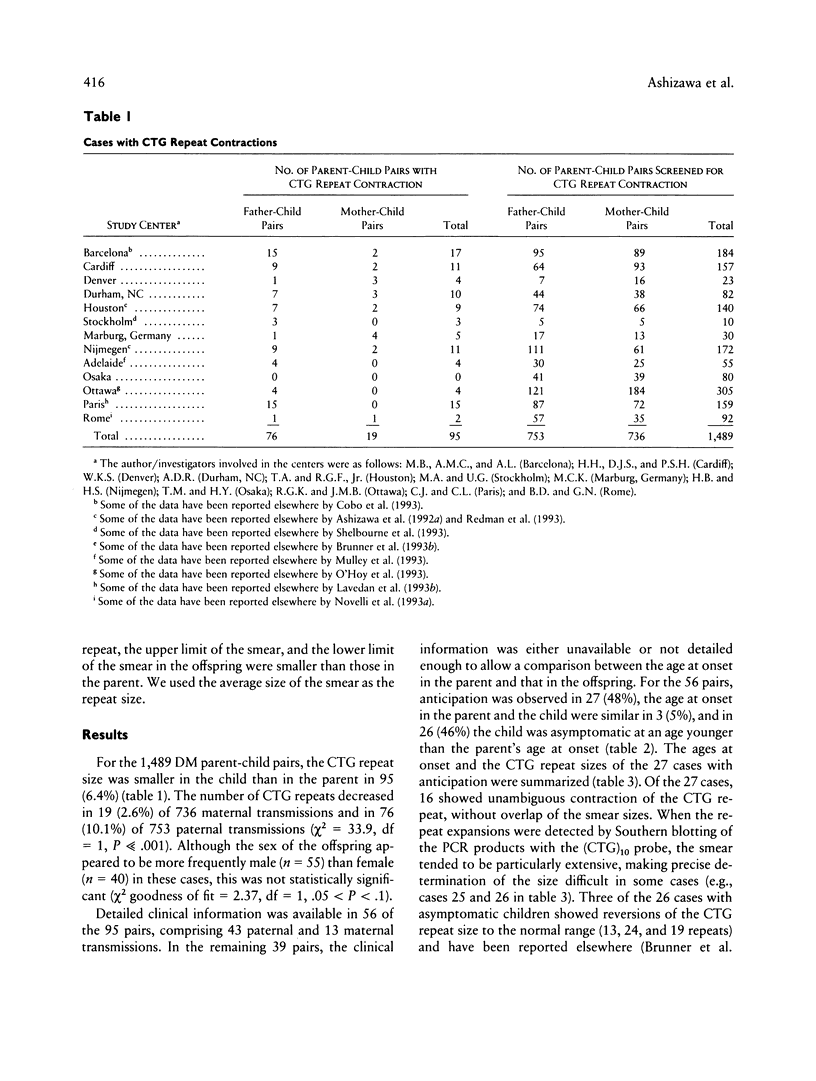
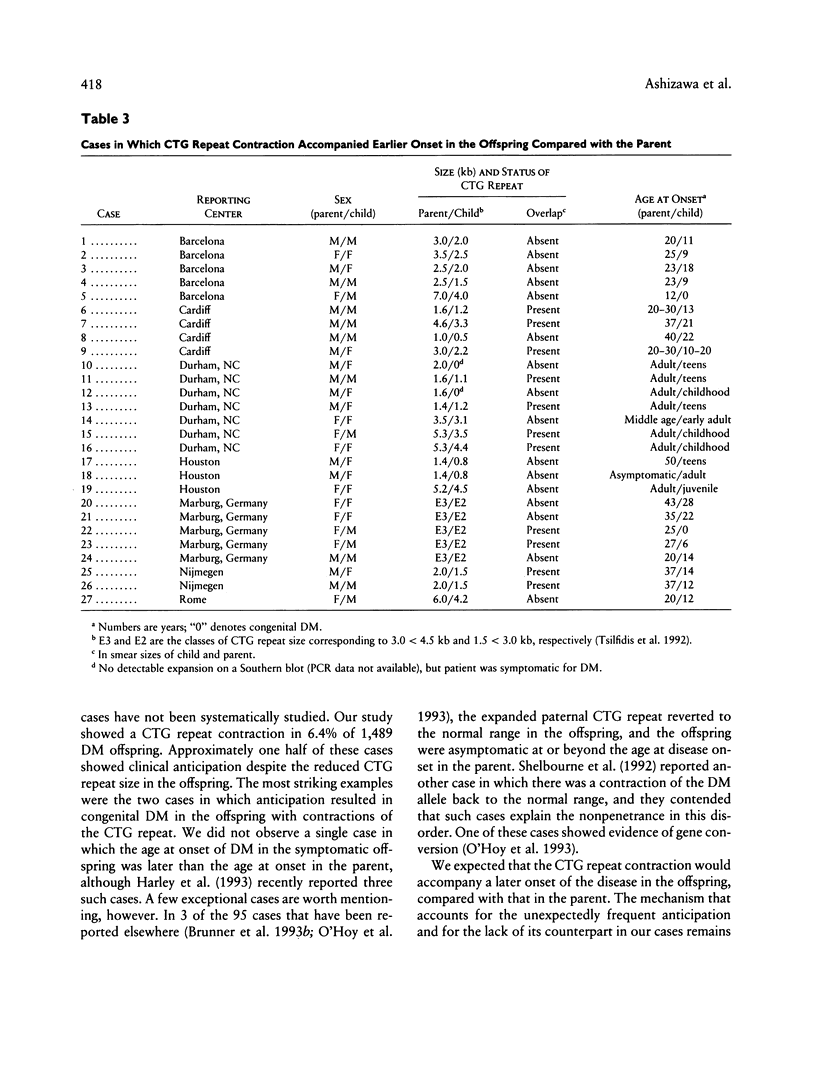
In myotonic dystrophy (DM), the size of a CTG repeat in the DM kinase gene generally increases in successive generations with clinical evidence of anticipation. However, there have also been cases with an intergenerational contraction of the repeat. We examined 1,489 DM parent-offspring pairs, of which 95 (6.4%) showed such contractions in peripheral blood leukocytes (PBL). In 56 of the 95 pairs, clinical data allowed an analysis of their anticipation status. It is surprising that anticipation occurred in 27 (48%) of these 56 pairs, while none clearly showed a later onset of DM in the symptomatic offspring. The contraction occurred in 76 (10%) of 753 paternal transmissions and in 19 (3%) of 736 maternal transmissions. Anticipation was observed more frequently in maternal (85%) than in paternal (37%) transmissions (P < .001). The parental repeat size correlated with the size of intergenerational contraction (r2 = .50, P « .001), and the slope of linear regression was steeper in paternal (–.62) than in maternal (–.30) transmissions (P « .001). Sixteen DM parents had multiple DM offspring with the CTG repeat contractions. This frequency was higher than the frequency expected from the probability of the repeat contractions (6.4%) and the size of DM sib population (1.54 DM offspring per DM parent, in 968 DM parents). We conclude that (1) intergenerational contraction of the CTG repeat in leukocyte DNA frequently accompanies apparent anticipation, especially when DM is maternally transmitted, and (2) the paternal origin of the repeat and the presence of the repeat contraction in a sibling increase the probability of the CTG repeat contraction.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich D., Lerer I., Pashut-Lavon I., Shmueli E., Raas-Rothschild A., Frydman M. Negative expansion of the myotonic dystrophy unstable sequence. Am J Hum Genet. 1993 Jun;52(6):1175–1181. [PMC free article] [PubMed] [Google Scholar]
- Anvret M., Ahlberg G., Grandell U., Hedberg B., Johnson K., Edström L. Larger expansions of the CTG repeat in muscle compared to lymphocytes from patients with myotonic dystrophy. Hum Mol Genet. 1993 Sep;2(9):1397–1400. doi: 10.1093/hmg/2.9.1397. [DOI] [PubMed] [Google Scholar]
- Ashizawa T., Dubel J. R., Dunne P. W., Dunne C. J., Fu Y. H., Pizzuti A., Caskey C. T., Boerwinkle E., Perryman M. B., Epstein H. F. Anticipation in myotonic dystrophy. II. Complex relationships between clinical findings and structure of the GCT repeat. Neurology. 1992 Oct;42(10):1877–1883. doi: 10.1212/wnl.42.10.1877. [DOI] [PubMed] [Google Scholar]
- Ashizawa T., Dubel J. R., Harati Y. Somatic instability of CTG repeat in myotonic dystrophy. Neurology. 1993 Dec;43(12):2674–2678. doi: 10.1212/wnl.43.12.2674. [DOI] [PubMed] [Google Scholar]
- Ashizawa T., Dunne C. J., Dubel J. R., Perryman M. B., Epstein H. F., Boerwinkle E., Hejtmancik J. F. Anticipation in myotonic dystrophy. I. Statistical verification based on clinical and haplotype findings. Neurology. 1992 Oct;42(10):1871–1877. doi: 10.1212/wnl.42.10.1871. [DOI] [PubMed] [Google Scholar]
- Ashizawa T., Dunne P. W., Ward P. A., Seltzer W. K., Richards C. S. Effects of the sex of myotonic dystrophy patients on the unstable triplet repeat in their affected offspring. Neurology. 1994 Jan;44(1):120–122. doi: 10.1212/wnl.44.1.120. [DOI] [PubMed] [Google Scholar]
- Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Chen C., Alleman J., Wormskamp N. G., Vooijs M. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992 Feb 6;355(6360):548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., Brüggenwirth H. T., Nillesen W., Jansen G., Hamel B. C., Hoppe R. L., de Die C. E., Höweler C. J., van Oost B. A., Wieringa B. Influence of sex of the transmitting parent as well as of parental allele size on the CTG expansion in myotonic dystrophy (DM). Am J Hum Genet. 1993 Nov;53(5):1016–1023. [PMC free article] [PubMed] [Google Scholar]
- Brunner H. G., Jansen G., Nillesen W., Nelen M. R., de Die C. E., Höweler C. J., van Oost B. A., Wieringa B., Ropers H. H., Smeets H. J. Brief report: reverse mutation in myotonic dystrophy. N Engl J Med. 1993 Feb 18;328(7):476–480. doi: 10.1056/NEJM199302183280705. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., Nillesen W., van Oost B. A., Jansen G., Wieringa B., Ropers H. H., Smeets H. J. Presymptomatic diagnosis of myotonic dystrophy. J Med Genet. 1992 Nov;29(11):780–784. doi: 10.1136/jmg.29.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton J., Shelbourne P., Davies J., Jones C., Van Tongeren T., Aslanidis C., de Jong P., Jansen G., Anvret M., Riley B. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature. 1992 Feb 6;355(6360):547–548. doi: 10.1038/355547a0. [DOI] [PubMed] [Google Scholar]
- Cobo A. M., Baiget M., López de Munain A., Poza J. J., Emparanza J. I., Johnson K. Sex-related difference in intergenerational expansion of myotonic dystrophy gene. Lancet. 1993 May 1;341(8853):1159–1160. doi: 10.1016/0140-6736(93)93186-5. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Friedman D. L., Richards S., Pearlman J. A., Gibbs R. A., Pizzuti A., Ashizawa T., Perryman M. B., Scarlato G., Fenwick R. G., Jr Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science. 1993 Apr 9;260(5105):235–238. doi: 10.1126/science.8469976. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Pizzuti A., Fenwick R. G., Jr, King J., Rajnarayan S., Dunne P. W., Dubel J., Nasser G. A., Ashizawa T., de Jong P. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992 Mar 6;255(5049):1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Rundle S. A., Crow S., Reardon W., Buckler A. J., Harper P. S., Housman D. E., Shaw D. J. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992 Feb 6;355(6360):545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Rundle S. A., MacMillan J. C., Myring J., Brook J. D., Crow S., Reardon W., Fenton I., Shaw D. J., Harper P. S. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am J Hum Genet. 1993 Jun;52(6):1164–1174. [PMC free article] [PubMed] [Google Scholar]
- Harley H. G., Rundle S. A., Reardon W., Myring J., Crow S., Brook J. D., Harper P. S., Shaw D. J. Unstable DNA sequence in myotonic dystrophy. Lancet. 1992 May 9;339(8802):1125–1128. doi: 10.1016/0140-6736(92)90729-m. [DOI] [PubMed] [Google Scholar]
- Harper P. S., Harley H. G., Reardon W., Shaw D. J. Anticipation in myotonic dystrophy: new light on an old problem. Am J Hum Genet. 1992 Jul;51(1):10–16. [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Radvanyi H., Lavedan C., Rabès J. P., Savoy D., Duros C., Johnson K., Junien C. Myotonic dystrophy: absence of CTG enlarged transcript in congenital forms, and low expression of the normal allele. Hum Mol Genet. 1993 Aug;2(8):1263–1266. doi: 10.1093/hmg/2.8.1263. [DOI] [PubMed] [Google Scholar]
- Hunter A. G., Jacob P., O'Hoy K., MacDonald I., Mettler G., Tsilfidis C., Korneluk R. G. Decrease in the size of the myotonic dystrophy CTG repeat during transmission from parent to child: implications for genetic counselling and genetic anticipation. Am J Med Genet. 1993 Feb 1;45(3):401–407. doi: 10.1002/ajmg.1320450330. [DOI] [PubMed] [Google Scholar]
- Hunter A., Tsilfidis C., Mettler G., Jacob P., Mahadevan M., Surh L., Korneluk R. The correlation of age of onset with CTG trinucleotide repeat amplification in myotonic dystrophy. J Med Genet. 1992 Nov;29(11):774–779. doi: 10.1136/jmg.29.11.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höweler C. J., Busch H. F., Geraedts J. P., Niermeijer M. F., Staal A. Anticipation in myotonic dystrophy: fact or fiction? Brain. 1989 Jun;112(Pt 3):779–797. doi: 10.1093/brain/112.3.779. [DOI] [PubMed] [Google Scholar]
- Jansen G., Bartolomei M., Kalscheuer V., Merkx G., Wormskamp N., Mariman E., Smeets D., Ropers H. H., Wieringa B. No imprinting involved in the expression of DM-kinase mRNAs in mouse and human tissues. Hum Mol Genet. 1993 Aug;2(8):1221–1227. doi: 10.1093/hmg/2.8.1221. [DOI] [PubMed] [Google Scholar]
- Koch M. C., Grimm T., Harley H. G., Harper P. S. Genetic risks for children of women with myotonic dystrophy. Am J Hum Genet. 1991 Jun;48(6):1084–1091. [PMC free article] [PubMed] [Google Scholar]
- Lavedan C., Hofmann-Radvanyi H., Rabes J. P., Roume J., Junien C. Different sex-dependent constraints in CTG length variation as explanation for congenital myotonic dystrophy. Lancet. 1993 Jan 23;341(8839):237–237. doi: 10.1016/0140-6736(93)90097-z. [DOI] [PubMed] [Google Scholar]
- Lavedan C., Hofmann-Radvanyi H., Shelbourne P., Rabes J. P., Duros C., Savoy D., Dehaupas I., Luce S., Johnson K., Junien C. Myotonic dystrophy: size- and sex-dependent dynamics of CTG meiotic instability, and somatic mosaicism. Am J Hum Genet. 1993 May;52(5):875–883. [PMC free article] [PubMed] [Google Scholar]
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992 Mar 6;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- Mulley J. C., Staples A., Donnelly A., Gedeon A. K., Hecht B. K., Nicholson G. A., Haan E. A., Sutherland G. R. Explanation for exclusive maternal origin for congenital form of myotonic dystrophy. Lancet. 1993 Jan 23;341(8839):236–237. doi: 10.1016/0140-6736(93)90096-y. [DOI] [PubMed] [Google Scholar]
- Novelli G., Gennarelli M., Menegazzo E., Mostacciuolo M. L., Pizzuti A., Fattorini C., Tessarolo D., Tomelleri G., Giacanelli M., Danieli G. A. (CTG)n triplet mutation and phenotype manifestations in myotonic dystrophy patients. Biochem Med Metab Biol. 1993 Aug;50(1):85–92. doi: 10.1006/bmmb.1993.1049. [DOI] [PubMed] [Google Scholar]
- Novelli G., Gennarelli M., Zelano G., Pizzuti A., Fattorini C., Caskey C. T., Dallapiccola B. Failure in detecting mRNA transcripts from the mutated allele in myotonic dystrophy muscle. Biochem Mol Biol Int. 1993 Feb;29(2):291–297. [PubMed] [Google Scholar]
- O'Hoy K. L., Tsilfidis C., Mahadevan M. S., Neville C. E., Barceló J., Hunter A. G., Korneluk R. G. Reduction in size of the myotonic dystrophy trinucleotide repeat mutation during transmission. Science. 1993 Feb 5;259(5096):809–812. doi: 10.1126/science.8094260. [DOI] [PubMed] [Google Scholar]
- Redman J. B., Fenwick R. G., Jr, Fu Y. H., Pizzuti A., Caskey C. T. Relationship between parental trinucleotide GCT repeat length and severity of myotonic dystrophy in offspring. JAMA. 1993 Apr 21;269(15):1960–1965. [PubMed] [Google Scholar]
- Sabouri L. A., Mahadevan M. S., Narang M., Lee D. S., Surh L. C., Korneluk R. G. Effect of the myotonic dystrophy (DM) mutation on mRNA levels of the DM gene. Nat Genet. 1993 Jul;4(3):233–238. doi: 10.1038/ng0793-233. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Chaudhary S., Rundle S. A., Crow S., Brook J. D., Harper P. S., Harley H. G. A study of DNA methylation in myotonic dystrophy. J Med Genet. 1993 Mar;30(3):189–192. doi: 10.1136/jmg.30.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelbourne P., Davies J., Buxton J., Anvret M., Blennow E., Bonduelle M., Schmedding E., Glass I., Lindenbaum R., Lane R. Direct diagnosis of myotonic dystrophy with a disease-specific DNA marker. N Engl J Med. 1993 Feb 18;328(7):471–475. doi: 10.1056/NEJM199302183280704. [DOI] [PubMed] [Google Scholar]
- Shelbourne P., Winqvist R., Kunert E., Davies J., Leisti J., Thiele H., Bachmann H., Buxton J., Williamson B., Johnson K. Unstable DNA may be responsible for the incomplete penetrance of the myotonic dystrophy phenotype. Hum Mol Genet. 1992 Oct;1(7):467–473. doi: 10.1093/hmg/1.7.467. [DOI] [PubMed] [Google Scholar]
- Tsilfidis C., MacKenzie A. E., Mettler G., Barceló J., Korneluk R. G. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nat Genet. 1992 Jun;1(3):192–195. doi: 10.1038/ng0692-192. [DOI] [PubMed] [Google Scholar]


