Abstract
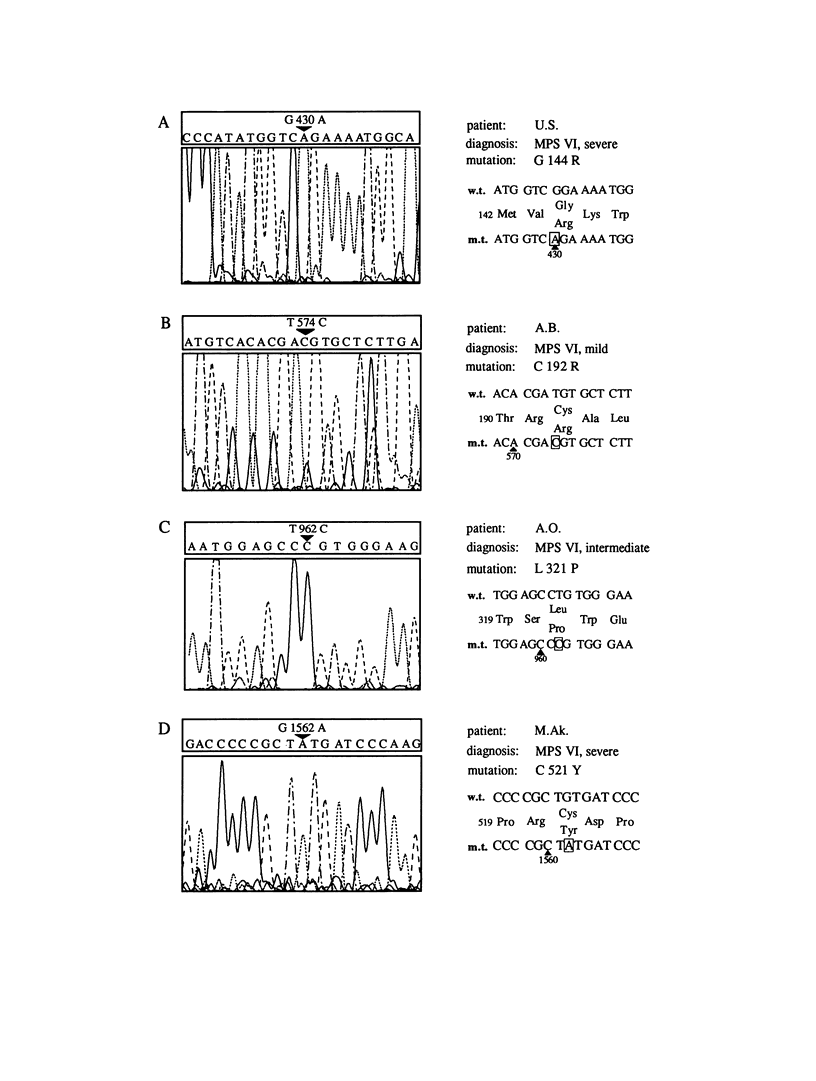
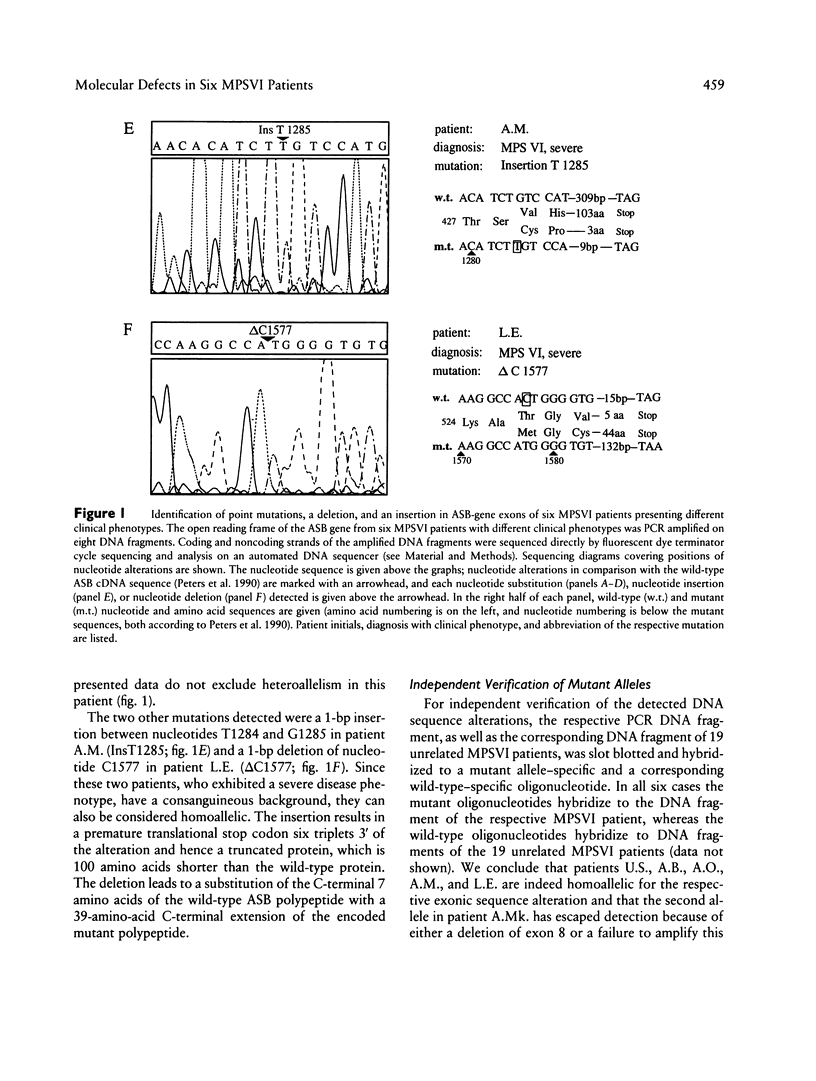
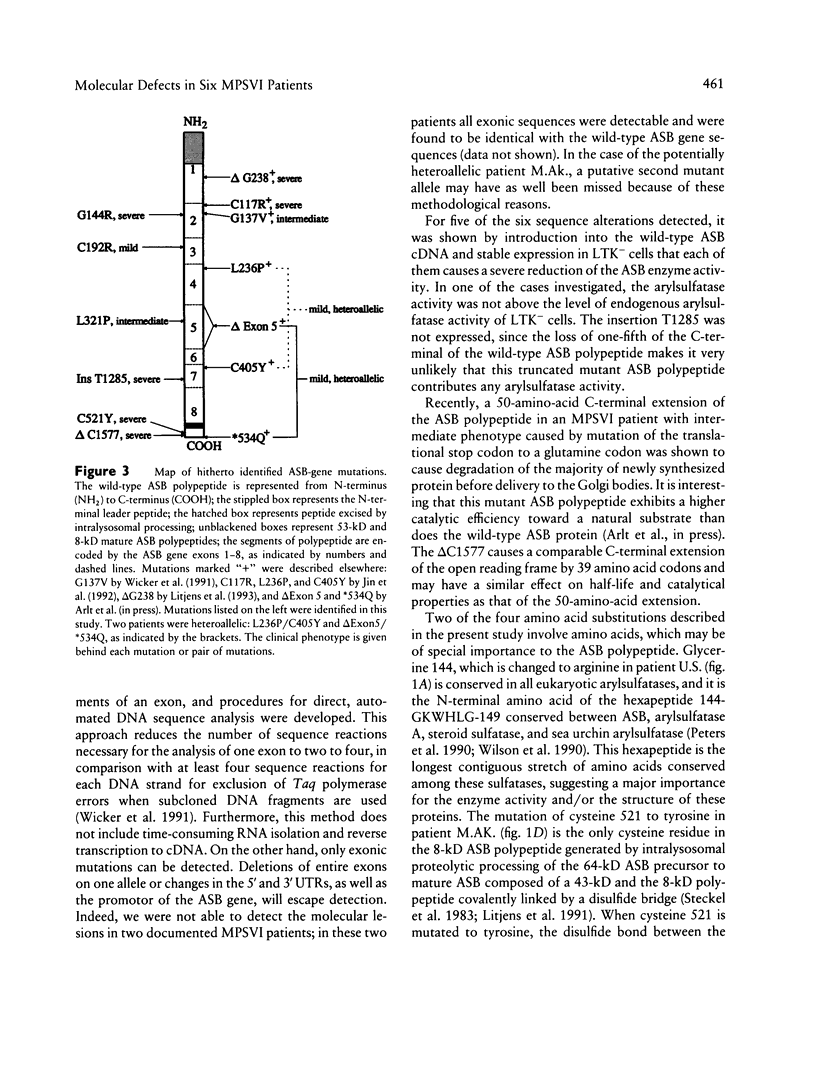
Mucopolysaccharidosis type VI, or Maroteaux-Lamy syndrome, is a lysosomal storage disorder caused by a deficiency of the enzyme arylsulfatase B (ASB), also known as N-acetylgalactosamine-4-sulfatase. Multiple clinical phenotypes of this autosomal recessively inherited disease have been described. Recent isolation and characterization of the human ASB gene facilitated the analysis of molecular defects underlying the different phenotypes. Conditions for PCR amplification of the entire open reading frame from genomic DNA and for subsequent direct automated DNA sequencing of the resulting DNA fragments were established. Besides two polymorphisms described elsewhere that cause methionine-for-valine substitutions in the arylsulfatase B gene, six new mutations in six patients were detected: four point mutations resulting in amino acid substitutions, a 1-bp deletion, and a 1-bp insertion. The point mutations were two G-to-A and two T-to-C transitions. The G-to-A transitions cause an arginine-for-glycine substitution at residue 144 in a homoallelic patient with a severe disease phenotype and a tyrosine-for-cysteine substitution at residue 521 in a potentially heteroallelic patient with the severe form of the disease. The T-to-C transitions cause an arginine-for-cysteine substitution at amino acid residue 192 in a homoallelic patient with mild symptoms and a proline-for-leucine substitution at amino acid 321 in a homoallelic patient with the intermediate form. The insertion between nucleotides T1284 and G1285 resulted in a loss of the 100 C-terminal amino acids of the wild-type protein and in the deletion of nucleotide C1577 in a 39-amino-acid C-terminal extension of the ASB polypeptide. Both mutations were detected in homoallelic patients with the severe form of the disease. Expression of mutant cDNAs encoding the four amino acid substitutions and the deletion resulted in severe reduction of both ASB protein levels and arylsulfatase enzyme activity in comparison with a wild-type control. The six mutations described in the present study were unique among 25 unrelated mucopolysaccharidosis VI patients, suggesting a broad molecular heterogeneity of the Maroteaux-Lamy syndrome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E., Roy A. B. The sulphatase of ox liver. XI. The isoelectric focussing of a purified preparation of sulphatase B. Biochim Biophys Acta. 1968 Oct 21;168(2):243–251. doi: 10.1016/0005-2795(68)90147-5. [DOI] [PubMed] [Google Scholar]
- Artelt P., Morelle C., Ausmeier M., Fitzek M., Hauser H. Vectors for efficient expression in mammalian fibroblastoid, myeloid and lymphoid cells via transfection or infection. Gene. 1988 Sep 7;68(2):213–219. doi: 10.1016/0378-1119(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Jin W. D., Desnick R. J., Schuchman E. H. A common polymorphism in the human arylsulfatase B (ARSB) gene at 5q13-q14. Nucleic Acids Res. 1991 Aug 11;19(15):4305–4305. doi: 10.1093/nar/19.15.4305-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W. D., Jackson C. E., Desnick R. J., Schuchman E. H. Mucopolysaccharidosis type VI: identification of three mutations in the arylsulfatase B gene of patients with the severe and mild phenotypes provides molecular evidence for genetic heterogeneity. Am J Hum Genet. 1992 Apr;50(4):795–800. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Litjens T., Morris C. P., Gibson G. J., Beckmann K. R., Hopwood J. J. Human N-acetylgalactosamine-4-sulphatase: protein maturation and isolation of genomic clones. Biochem Int. 1991 May;24(2):209–215. [PubMed] [Google Scholar]
- Litjens T., Morris C. P., Robertson E. F., Peters C., von Figura K., Hopwood J. J. An N-acetylgalactosamine-4-sulfatase mutation (delta G238) results in a severe Maroteaux-Lamy phenotype. Hum Mutat. 1992;1(5):397–402. doi: 10.1002/humu.1380010509. [DOI] [PubMed] [Google Scholar]
- Lyons K., Graycar J. L., Lee A., Hashmi S., Lindquist P. B., Chen E. Y., Hogan B. L., Derynck R. Vgr-1, a mammalian gene related to Xenopus Vg-1, is a member of the transforming growth factor beta gene superfamily. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4554–4558. doi: 10.1073/pnas.86.12.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R., Arbogast B., Dorfman A. Deficiency of chondroitin sulfate N-acetylgalactosamine 4-sulfate sulfatase in Maroteaux-Lamy syndrome. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1450–1457. doi: 10.1016/s0006-291x(74)80446-8. [DOI] [PubMed] [Google Scholar]
- Modaressi S., Rupp K., von Figura K., Peters C. Structure of the human arylsulfatase B gene. Biol Chem Hoppe Seyler. 1993 May;374(5):327–335. doi: 10.1515/bchm3.1993.374.1-6.327. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. F., Cantz M., Spranger J. Maroteaux-Lamy disease (mucopolysaccharidosis VI), subtype A: deficiency of a N-acetylgalactosamine-4-sulfatase. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1170–1177. doi: 10.1016/0006-291x(74)90435-5. [DOI] [PubMed] [Google Scholar]
- Peters C., Schmidt B., Rommerskirch W., Rupp K., Zühlsdorf M., Vingron M., Meyer H. E., Pohlmann R., von Figura K. Phylogenetic conservation of arylsulfatases. cDNA cloning and expression of human arylsulfatase B. J Biol Chem. 1990 Feb 25;265(6):3374–3381. [PubMed] [Google Scholar]
- Schuchman E. H., Jackson C. E., Desnick R. J. Human arylsulfatase B: MOPAC cloning, nucleotide sequence of a full-length cDNA, and regions of amino acid identity with arylsulfatases A and C. Genomics. 1990 Jan;6(1):149–158. doi: 10.1016/0888-7543(90)90460-c. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Steckel F., Hasilik A., von Figura K. Biosynthesis and maturation of arylsulfatase B in normal and mutant cultured human fibroblasts. J Biol Chem. 1983 Dec 10;258(23):14322–14326. [PubMed] [Google Scholar]
- Van Biervliet J. P., Van Leeuwen E. F., Abeling N. G., De Jonge H. F., Liem K. O., Wadman S. K. Un cas de maladie de maroteaux-lamy decouvert precocement. Arch Fr Pediatr. 1977 Apr;34(4):362–370. [PubMed] [Google Scholar]
- Wicker G., Prill V., Brooks D., Gibson G., Hopwood J., von Figura K., Peters C. Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). An intermediate clinical phenotype caused by substitution of valine for glycine at position 137 of arylsulfatase B. J Biol Chem. 1991 Nov 15;266(32):21386–21391. [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wilson P. J., Morris C. P., Anson D. S., Occhiodoro T., Bielicki J., Clements P. R., Hopwood J. J. Hunter syndrome: isolation of an iduronate-2-sulfatase cDNA clone and analysis of patient DNA. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8531–8535. doi: 10.1073/pnas.87.21.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Hasilik A. Juvenile and adult metachromatic leukodystrophy: partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6066–6070. doi: 10.1073/pnas.80.19.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]



