Abstract
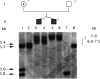
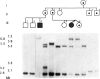
The fragile X phenotype has been found, in the majority of cases, to be due to the expansion of a CGG repeat in the 5'-UTR region of the FMR-1 gene, accompanied by methylation of the adjacent CpG island and inactivation of the FMR-1 gene. Although several important aspects of the genetics of fragile X have been resolved, it remains to be elucidated at which stage in development the transition from the premutation to the full mutation occurs. We present two families in which discordance between two sets of MZ twins illustrates two important genetic points. In one family, two affected MZ brothers differed in the number of CGG repeats, demonstrating in vivo mitotic instability of this CGG repeat and suggesting that the transition to the full mutation occurred postzygotically. In the second family, two MZ sisters had the same number of repeats, but only one was mentally retarded. When the methylation status of the FMR-1 CpG island was studied, we found that the majority of normal chromosomes had been inactivated in the affected twin, thus leading to the expression of the fragile X phenotype.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs B. A., Nussbaum R. L. Two anonymous X-specific human sequences detecting restriction fragment length polymorphisms in region Xq26----qter. Somat Cell Mol Genet. 1984 Nov;10(6):607–613. doi: 10.1007/BF01535226. [DOI] [PubMed] [Google Scholar]
- Brook J. D. Retreat of the triplet repeat? Nat Genet. 1993 Apr;3(4):279–281. doi: 10.1038/ng0493-279. [DOI] [PubMed] [Google Scholar]
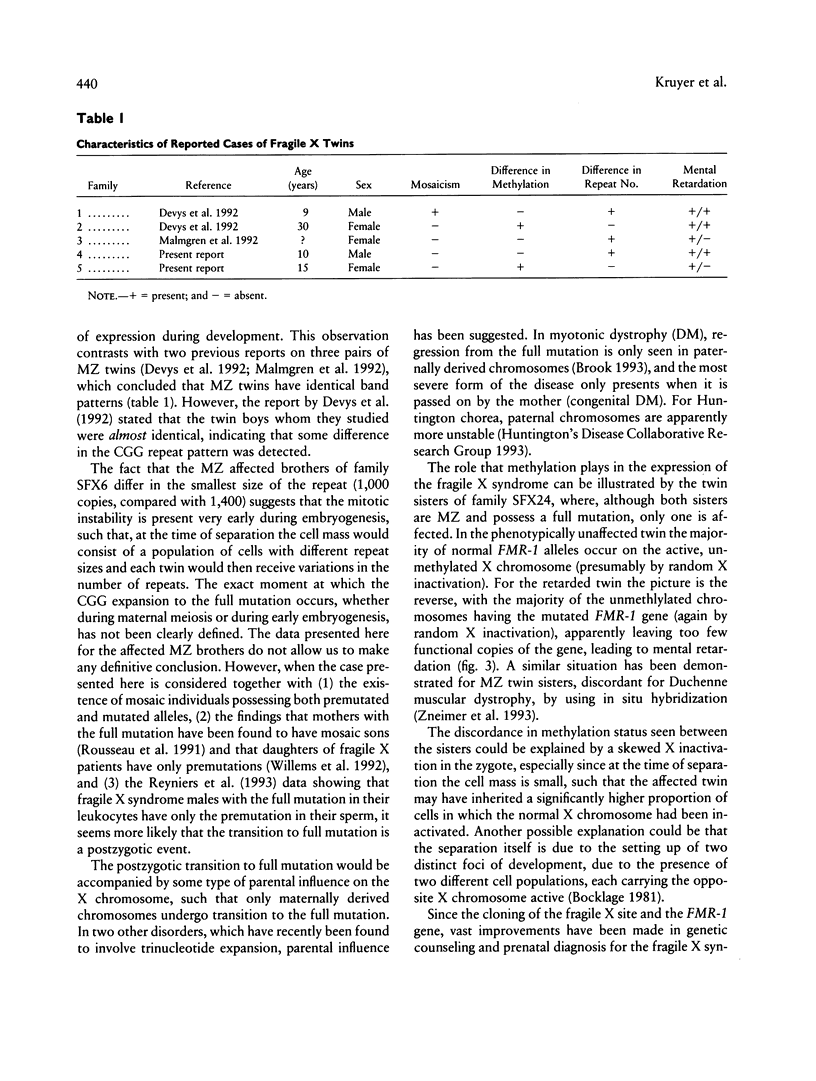
- Devys D., Biancalana V., Rousseau F., Boué J., Mandel J. L., Oberlé I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. 1992 Apr 15-May 1Am J Med Genet. 43(1-2):208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Kioschis P., Monaco A. P., Gross B., Korn B., Williams S. V., Sheer D., Heitz D., Oberle I., Toniolo D. Molecular cloning and analysis of the fragile X region in man. Nucleic Acids Res. 1991 May 25;19(10):2567–2572. doi: 10.1093/nar/19.10.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Fuentes J. J., Banchs I., Volpini V., Estivill X. Genetic variation of microsatellite markers D1S117, D6S89, D11S35, APOC2, and D21S168 in the Spanish population. Int J Legal Med. 1993;105(5):271–277. doi: 10.1007/BF01370384. [DOI] [PubMed] [Google Scholar]
- Heitz D., Rousseau F., Devys D., Saccone S., Abderrahim H., Le Paslier D., Cohen D., Vincent A., Toniolo D., Della Valle G. Isolation of sequences that span the fragile X and identification of a fragile X-related CpG island. Science. 1991 Mar 8;251(4998):1236–1239. doi: 10.1126/science.2006411. [DOI] [PubMed] [Google Scholar]
- Kremer E. J., Pritchard M., Lynch M., Yu S., Holman K., Baker E., Warren S. T., Schlessinger D., Sutherland G. R., Richards R. I. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991 Jun 21;252(5013):1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- Malmgren H., Steén-Bondeson M. L., Gustavson K. H., Seémanova E., Holmgren G., Oberlé I., Mandel J. L., Pettersson U., Dahl N. Methylation and mutation patterns in the fragile X syndrome. 1992 Apr 15-May 1Am J Med Genet. 43(1-2):268–278. doi: 10.1002/ajmg.1320430142. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlé I., Rousseau F., Heitz D., Kretz C., Devys D., Hanauer A., Boué J., Bertheas M. F., Mandel J. L. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991 May 24;252(5009):1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Patterson M., Bell M., Kress W., Davies K. E., Froster-Iskenius U. Linkage studies in a large fragile X family. Am J Hum Genet. 1988 Nov;43(5):684–688. [PMC free article] [PubMed] [Google Scholar]
- Reyniers E., Vits L., De Boulle K., Van Roy B., Van Velzen D., de Graaff E., Verkerk A. J., Jorens H. Z., Darby J. K., Oostra B. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993 Jun;4(2):143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boué J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M. F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991 Dec 12;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Jacobs P. A., Morton N. E., Froster-Iskenius U., Howard-Peebles P. N., Nielsen K. B., Partington M. W., Sutherland G. R., Turner G., Watson M. Further segregation analysis of the fragile X syndrome with special reference to transmitting males. Hum Genet. 1985;69(4):289–299. doi: 10.1007/BF00291644. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Morton N. E., Jacobs P. A., Turner G. The marker (X) syndrome: a cytogenetic and genetic analysis. Ann Hum Genet. 1984 Jan;48(Pt 1):21–37. doi: 10.1111/j.1469-1809.1984.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Sutherland G. R. Heritable fragile sites on human chromosomes I. Factors affecting expression in lymphocyte culture. Am J Hum Genet. 1979 Mar;31(2):125–135. [PMC free article] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Willems P. J., Van Roy B., De Boulle K., Vits L., Reyniers E., Beck O., Dumon J. E., Verkerk A., Oostra B. Segregation of the fragile X mutation from an affected male to his normal daughter. Hum Mol Genet. 1992 Oct;1(7):511–515. doi: 10.1093/hmg/1.7.511. [DOI] [PubMed] [Google Scholar]
- Yu S., Pritchard M., Kremer E., Lynch M., Nancarrow J., Baker E., Holman K., Mulley J. C., Warren S. T., Schlessinger D. Fragile X genotype characterized by an unstable region of DNA. Science. 1991 May 24;252(5009):1179–1181. doi: 10.1126/science.252.5009.1179. [DOI] [PubMed] [Google Scholar]
- Zneimer S. M., Schneider N. R., Richards C. S. In situ hybridization shows direct evidence of skewed X inactivation in one of monozygotic twin females manifesting Duchenne muscular dystrophy. Am J Med Genet. 1993 Mar 1;45(5):601–605. doi: 10.1002/ajmg.1320450517. [DOI] [PubMed] [Google Scholar]