Abstract
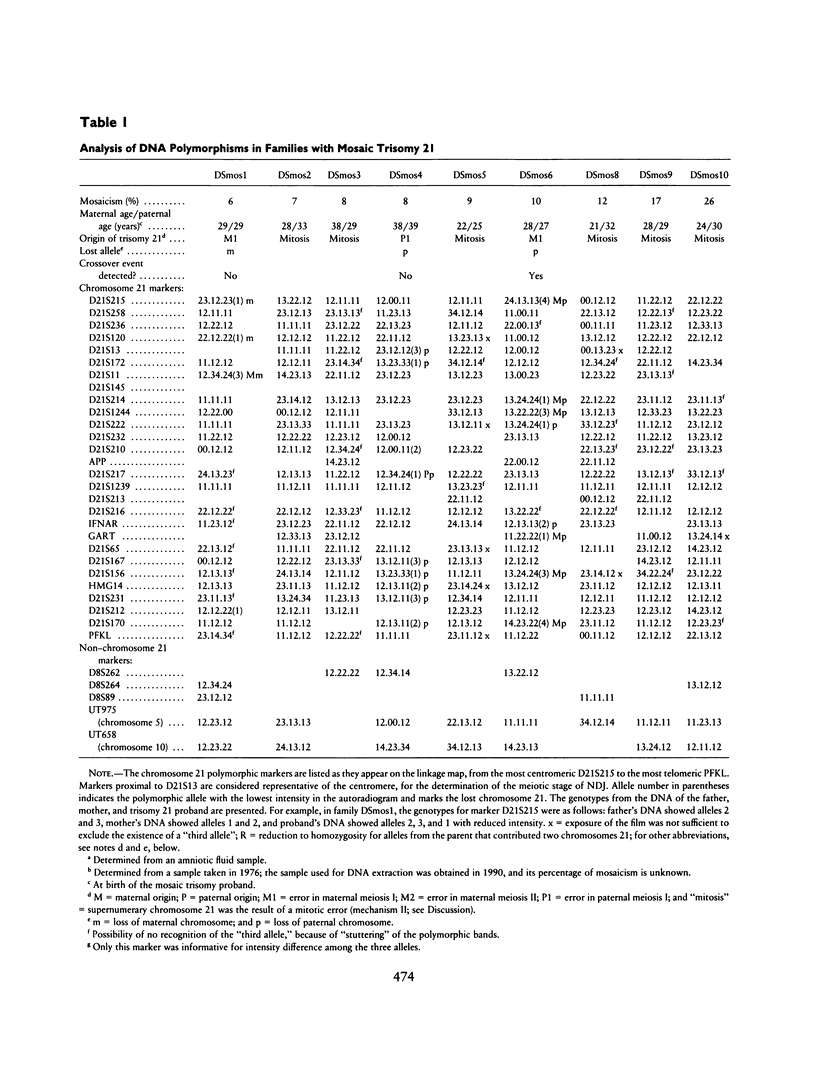
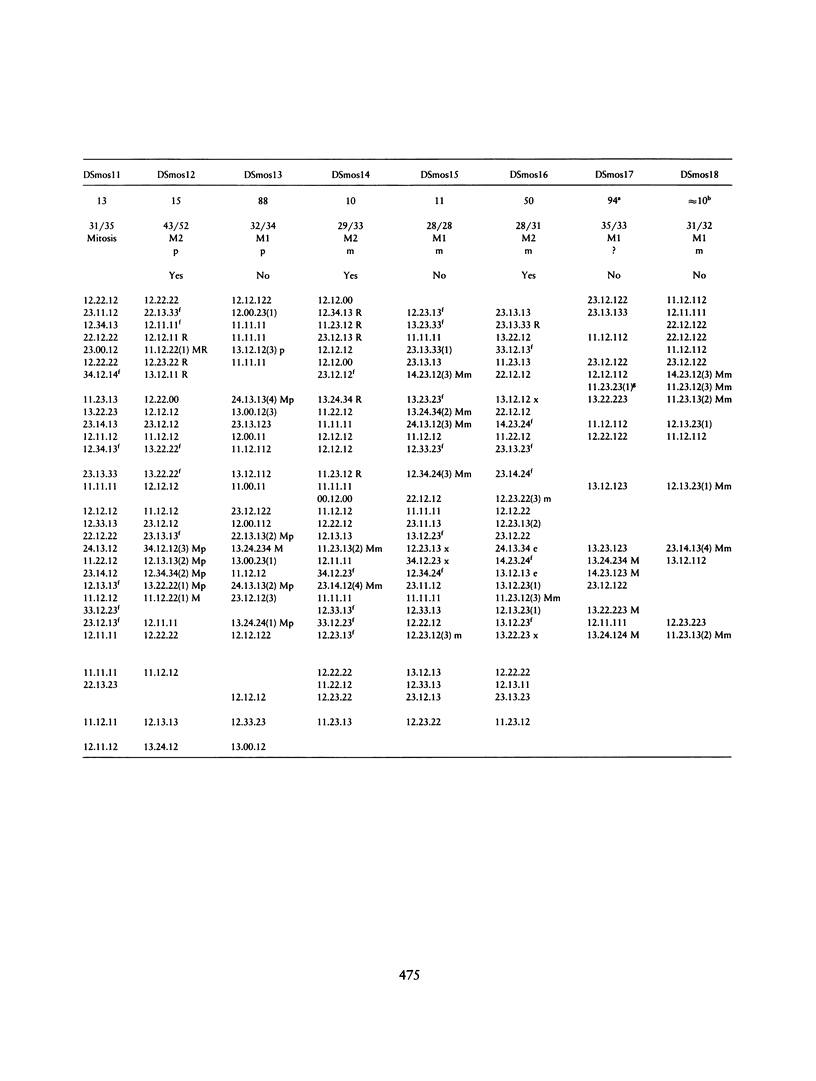
In order to investigate the mechanism(s) underlying mosaicism for trisomy 21, we genotyped 17 families with mosaic trisomy 21 probands, using 28 PCR-detectable DNA polymorphic markers that map in the pericentromeric region and long arm of chromosome 21. The percentage of cells with trisomy 21 in the probands' blood lymphocytes was 6%-94%. There were two classes of autoradiographic results: In class I, a "third allele" of lower intensity was detected in the proband's DNA for at least two chromosome 21 markers. The interpretation of this result was that the proband had inherited three chromosomes 21 after meiotic nondisjunction (NDJ) (trisomy 21 zygote) and subsequently lost one because of mitotic (somatic) error, the lost chromosome 21 being that with the lowest-intensity polymorphic allele. The parental origin and the meiotic stage of NDJ could also be determined. In class II, a "third allele" was never detected. In these cases, the mosaicism probably occurred either by a postzygotic, mitotic error in a normal zygote that followed a normal meiosis (class IIA mechanism); by premeiotic, mitotic NDJ yielding an aneusomic zygote after meiosis, and subsequent mitotic loss (class IIB mechanism); or by a meiosis II error with lack of crossover in the preceding meiosis I, followed by mitotic loss after fertilization (class IIC mechanism). Among class II mechanisms, the most likely is mechanism IIA, while IIC is the least likely. There were 10 cases of class I and 7 cases of class II results.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

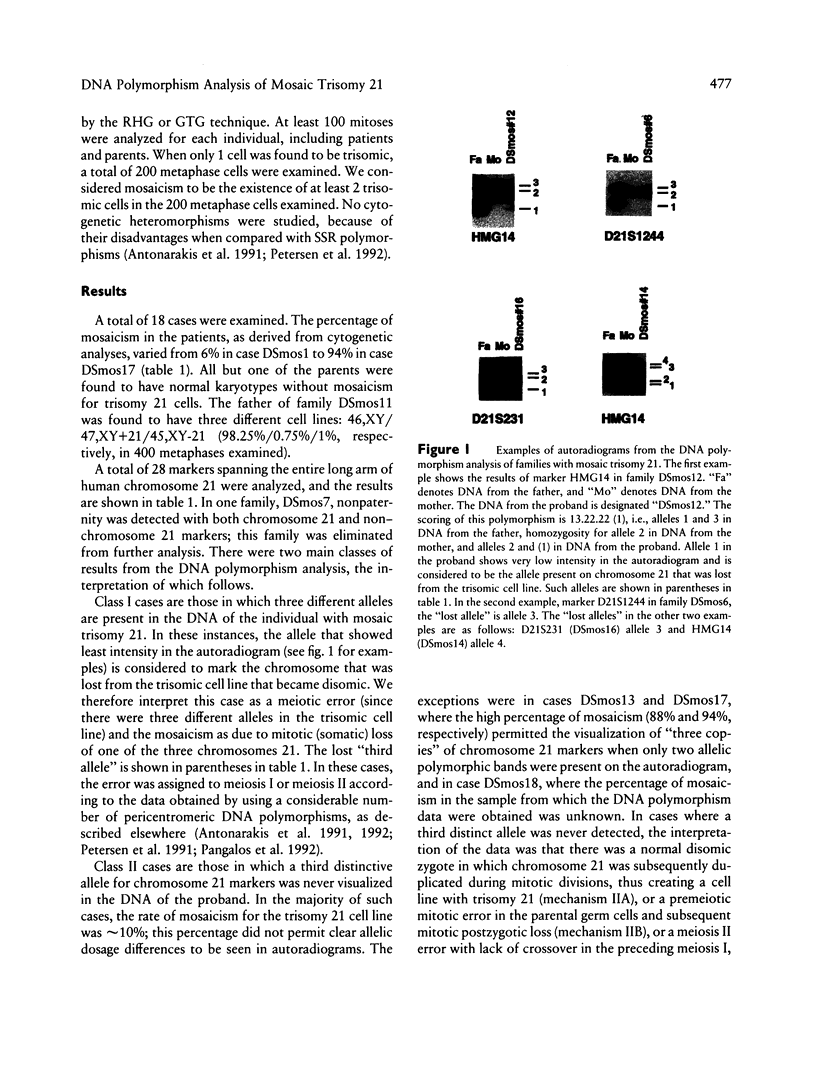




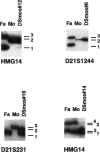
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Avramopoulos D., Blouin J. L., Talbot C. C., Jr, Schinzel A. A. Mitotic errors in somatic cells cause trisomy 21 in about 4.5% of cases and are not associated with advanced maternal age. Nat Genet. 1993 Feb;3(2):146–150. doi: 10.1038/ng0293-146. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Blouin J. L., Maher J., Avramopoulos D., Thomas G., Talbot C. C., Jr Maternal uniparental disomy for human chromosome 14, due to loss of a chromosome 14 from somatic cells with t(13;14) trisomy 14. Am J Hum Genet. 1993 Jun;52(6):1145–1152. [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E. Human chromosome 21: genome mapping and exploration, circa 1993. Trends Genet. 1993 Apr;9(4):142–148. doi: 10.1016/0168-9525(93)90210-9. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E. Parental origin of the extra chromosome in trisomy 21 as indicated by analysis of DNA polymorphisms. Down Syndrome Collaborative Group. N Engl J Med. 1991 Mar 28;324(13):872–876. doi: 10.1056/NEJM199103283241302. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Petersen M. B., McInnis M. G., Adelsberger P. A., Schinzel A. A., Binkert F., Pangalos C., Raoul O., Slaugenhaupt S. A., Hafez M. The meiotic stage of nondisjunction in trisomy 21: determination by using DNA polymorphisms. Am J Hum Genet. 1992 Mar;50(3):544–550. [PMC free article] [PubMed] [Google Scholar]
- Blouin J. L., Avramopoulos D., Pangalos C., Antonarakis S. E. Normal phenotype with paternal uniparental isodisomy for chromosome 21. Am J Hum Genet. 1993 Nov;53(5):1074–1078. [PMC free article] [PubMed] [Google Scholar]
- Créau-Goldberg N., Gegonne A., Delabar J., Cochet C., Cabanis M. O., Stehelin D., Turleau C., de Grouchy J. Maternal origin of a de novo balanced t(21q21q) identified by ets-2 polymorphism. Hum Genet. 1987 Aug;76(4):396–398. doi: 10.1007/BF00272452. [DOI] [PubMed] [Google Scholar]
- Dagna Bricarelli F., Pierluigi M., Grasso M., Strigini P., Perroni L. Origin of extra chromosome 21 in 343 families: cytogenetic and molecular approaches. Am J Med Genet Suppl. 1990;7:129–132. doi: 10.1002/ajmg.1320370726. [DOI] [PubMed] [Google Scholar]
- Juberg R. C., Holliday D. J., Hennessy V. S. Familial sex chromosomal mosaicism. Am J Med Genet. 1990 Sep;37(1):15–17. doi: 10.1002/ajmg.1320370105. [DOI] [PubMed] [Google Scholar]
- LEJEUNE J., GAUTIER M., TURPIN R. Etude des chromosomes somatiques de neuf enfants mongoliens. C R Hebd Seances Acad Sci. 1959 Mar 16;248(11):1721–1722. [PubMed] [Google Scholar]
- McInnis M. G., Chakravarti A., Blaschak J., Petersen M. B., Sharma V., Avramopoulos D., Blouin J. L., König U., Brahe C., Matise T. C. A linkage map of human chromosome 21:43 PCR markers at average intervals of 2.5 cM. Genomics. 1993 Jun;16(3):562–571. doi: 10.1006/geno.1993.1231. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M. Down syndrome: cytogenetical epidemiology. Hereditas. 1977;86(1):45–50. doi: 10.1111/j.1601-5223.1977.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Niikawa N., Kajii T. The origin of mosaic Down syndrome: four cases with chromosome markers. Am J Hum Genet. 1984 Jan;36(1):123–130. [PMC free article] [PubMed] [Google Scholar]
- Pangalos C. G., Talbot C. C., Jr, Lewis J. G., Adelsberger P. A., Petersen M. B., Serre J. L., Rethoré M. O., de Blois M. C., Parent P., Schinzel A. A. DNA polymorphism analysis in families with recurrence of free trisomy 21. Am J Hum Genet. 1992 Nov;51(5):1015–1027. [PMC free article] [PubMed] [Google Scholar]
- Petersen M. B., Schinzel A. A., Binkert F., Tranebjaerg L., Mikkelsen M., Collins F. A., Economou E. P., Antonarakis S. E. Use of short sequence repeat DNA polymorphisms after PCR amplification to detect the parental origin of the additional chromosome 21 in Down syndrome. Am J Hum Genet. 1991 Jan;48(1):65–71. [PMC free article] [PubMed] [Google Scholar]
- Peterson M. B., Frantzen M., Antonarakis S. E., Warren A. C., Van Broeckhoven C., Chakravarti A., Cox T. K., Lund C., Olsen B., Poulsen H. Comparative study of microsatellite and cytogenetic markers for detecting the origin of the nondisjoined chromosome 21 in Down syndrome. Am J Hum Genet. 1992 Sep;51(3):516–525. [PMC free article] [PubMed] [Google Scholar]
- Richards B. W. Mosaic mongolism. J Ment Defic Res. 1969 Mar;13(1):66–83. doi: 10.1111/j.1365-2788.1969.tb01067.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Takaesu N., Freeman S. B., Grantham M., Phillips C., Blackston R. D., Jacobs P. A., Cockwell A. E., Freeman V., Uchida I. Trisomy 21: association between reduced recombination and nondisjunction. Am J Hum Genet. 1991 Sep;49(3):608–620. [PMC free article] [PubMed] [Google Scholar]
- Taylor A. I. Cell selection in vivo in normal-G trisomic mosaics. Nature. 1968 Sep 7;219(5158):1028–1030. doi: 10.1038/2191028a0. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]