Abstract
Two hypotheses are capable of explaining nonrandom loss of one parent's alleles at tumor suppressor loci in sporadic cases of several pediatric cancers, including retinoblastoma—namely, preferential germ-line mutation or chromosome imprinting. We have examined 74 cases of sporadic retinoblastoma for tumors in which at least two genetic events—loss of heterozygosity for chromosome 13q markers and formation of an isochromosome 6p—have occurred. Sixteen cases were found to contain both events. In 13 of 16 such tumors, the chromosomes 13q that were lost and chromosomes 6p that were duplicated are derived from the same parent. These data may be explained within the framework of the genome imprinting model but are not predicted by preferential germ-line mutation.
Full text
PDF


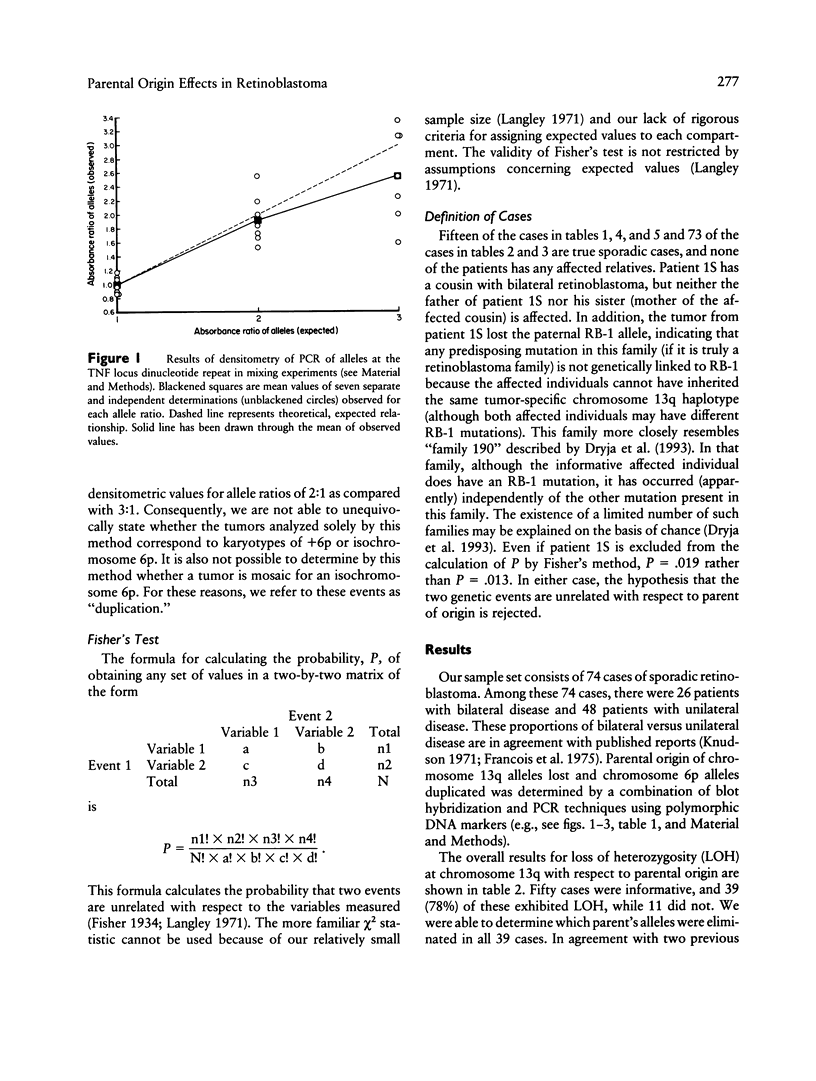




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Barlow D. P., Stöger R., Herrmann B. G., Saito K., Schweifer N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature. 1991 Jan 3;349(6304):84–87. doi: 10.1038/349084a0. [DOI] [PubMed] [Google Scholar]
- Blanquet V., Turleau C., de Grouchy J., Creau-Goldberg N. Physical map around the retinoblastoma gene: possible genomic imprinting suggested by NruI digestion. Genomics. 1991 Jun;10(2):350–355. doi: 10.1016/0888-7543(91)90319-a. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Murphree A. L., Shull M. M., Benedict W. F., Sparkes R. S., Kock E., Nordenskjold M. Prediction of familial predisposition to retinoblastoma. N Engl J Med. 1986 May 8;314(19):1201–1207. doi: 10.1056/NEJM198605083141901. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Mukai S., Petersen R., Rapaport J. M., Walton D., Yandell D. W. Parental origin of mutations of the retinoblastoma gene. Nature. 1989 Jun 15;339(6225):556–558. doi: 10.1038/339556a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forejt J., Gregorová S. Genetic analysis of genomic imprinting: an Imprintor-1 gene controls inactivation of the paternal copy of the mouse Tme locus. Cell. 1992 Aug 7;70(3):443–450. doi: 10.1016/0092-8674(92)90168-c. [DOI] [PubMed] [Google Scholar]
- François J., Matton M. T., De Bie S., Tanaka Y., Vandenbulcke D. Genesis and genetics of retinoblastoma. Ophthalmologica. 1975;170(5):405–425. doi: 10.1159/000307248. [DOI] [PubMed] [Google Scholar]
- Grundy P., Koufos A., Morgan K., Li F. P., Meadows A. T., Cavenee W. K. Familial predisposition to Wilms' tumour does not map to the short arm of chromosome 11. Nature. 1988 Nov 24;336(6197):374–376. doi: 10.1038/336374a0. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Greger V., Becher R., Passarge E. Mechanism of i(6p) formation in retinoblastoma tumor cells. Cancer Genet Cytogenet. 1989 Jan;37(1):95–102. doi: 10.1016/0165-4608(89)90079-4. [DOI] [PubMed] [Google Scholar]
- Huff V., Compton D. A., Chao L. Y., Strong L. C., Geiser C. F., Saunders G. F. Lack of linkage of familial Wilms' tumour to chromosomal band 11p13. Nature. 1988 Nov 24;336(6197):377–378. doi: 10.1038/336377a0. [DOI] [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova A., Sapienza C. The genetics of retinoblastoma, revisited. Am J Hum Genet. 1994 Feb;54(2):264–273. [PMC free article] [PubMed] [Google Scholar]
- Nedospasov S. A., Udalova I. A., Kuprash D. V., Turetskaya R. L. DNA sequence polymorphism at the human tumor necrosis factor (TNF) locus. Numerous TNF/lymphotoxin alleles tagged by two closely linked microsatellites in the upstream region of the lymphotoxin (TNF-beta) gene. J Immunol. 1991 Aug 1;147(3):1053–1059. [PubMed] [Google Scholar]
- Potluri V. R., Helson L., Ellsworth R. M., Reid T., Gilbert F. Chromosomal abnormalities in human retinoblastoma. A review. Cancer. 1986 Aug 1;58(3):663–671. doi: 10.1002/1097-0142(19860801)58:3<663::aid-cncr2820580311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Reik W., Surani M. A. Cancer genetics. Genomic imprinting and embryonal tumours. Nature. 1989 Mar 9;338(6211):112–113. doi: 10.1038/338112a0. [DOI] [PubMed] [Google Scholar]
- Sapienza C., Paquette J., Pannunzio P., Albrechtson S., Morgan K. The polar-lethal Ovum mutant gene maps to the distal portion of mouse chromosome 11. Genetics. 1992 Sep;132(1):241–246. doi: 10.1093/genetics/132.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer H., te Meerman G. J., Kruize Y. C., van den Berg A. H., Penninga D. P., Tan K. E., der Kinderen D. J., Buys C. H. Linkage analysis of families with hereditary retinoblastoma: nonpenetrance of mutation, revealed by combined use of markers within and flanking the RB1 gene. Am J Hum Genet. 1989 Aug;45(2):252–260. [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. T., Chao L. Y., Dao D. D., Strong L. C., Pathak S., Riccardi V., Lewis W. H., Saunders G. F. Nonrandom loss of maternal chromosome 11 alleles in Wilms tumors. Am J Hum Genet. 1987 May;40(5):413–420. [PMC free article] [PubMed] [Google Scholar]
- Scrable H., Cavenee W., Ghavimi F., Lovell M., Morgan K., Sapienza C. A model for embryonal rhabdomyosarcoma tumorigenesis that involves genome imprinting. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7480–7484. doi: 10.1073/pnas.86.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J., Gallie B. L., Phillips R. A. A detailed analysis of chromosomal changes in heritable and non-heritable retinoblastoma. Hum Genet. 1985;70(4):291–301. doi: 10.1007/BF00295364. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Toguchida J., Ishizaki K., Sasaki M. S., Nakamura Y., Ikenaga M., Kato M., Sugimot M., Kotoura Y., Yamamuro T. Preferential mutation of paternally derived RB gene as the initial event in sporadic osteosarcoma. Nature. 1989 Mar 9;338(6211):156–158. doi: 10.1038/338156a0. [DOI] [PubMed] [Google Scholar]
- Turleau C., de Grouchy J., Chavin-Colin F., Junien C., Séger J., Schlienger P., Leblanc A., Haye C. Cytogenetic forms of retinoblastoma: their incidence in a survey of 66 patients. Cancer Genet Cytogenet. 1985 Apr 15;16(4):321–334. doi: 10.1016/0165-4608(85)90240-7. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Brown K. W., Mott M. G., Maitland N. J. Maternal allele loss in Wilms' tumour. Lancet. 1989 Feb 4;1(8632):283–284. doi: 10.1016/s0140-6736(89)91300-7. [DOI] [PubMed] [Google Scholar]
- Zhu X. P., Dunn J. M., Phillips R. A., Goddard A. D., Paton K. E., Becker A., Gallie B. L. Preferential germline mutation of the paternal allele in retinoblastoma. Nature. 1989 Jul 27;340(6231):312–313. doi: 10.1038/340312a0. [DOI] [PubMed] [Google Scholar]