Abstract
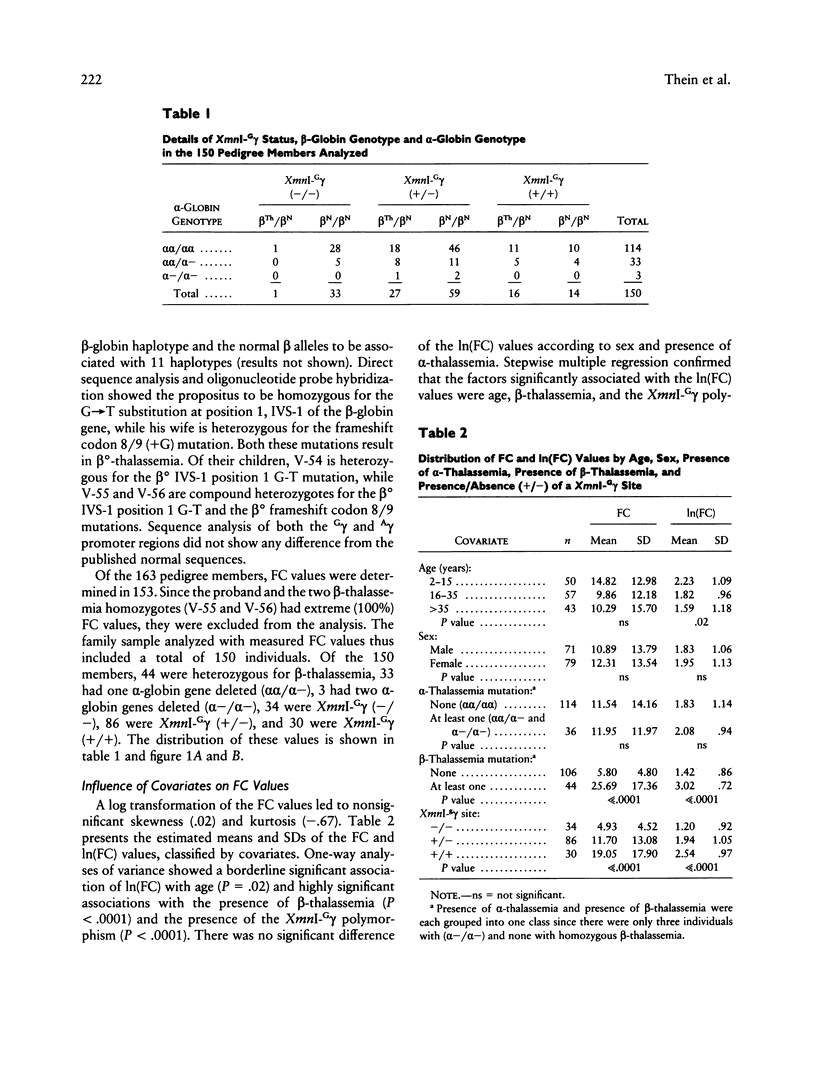
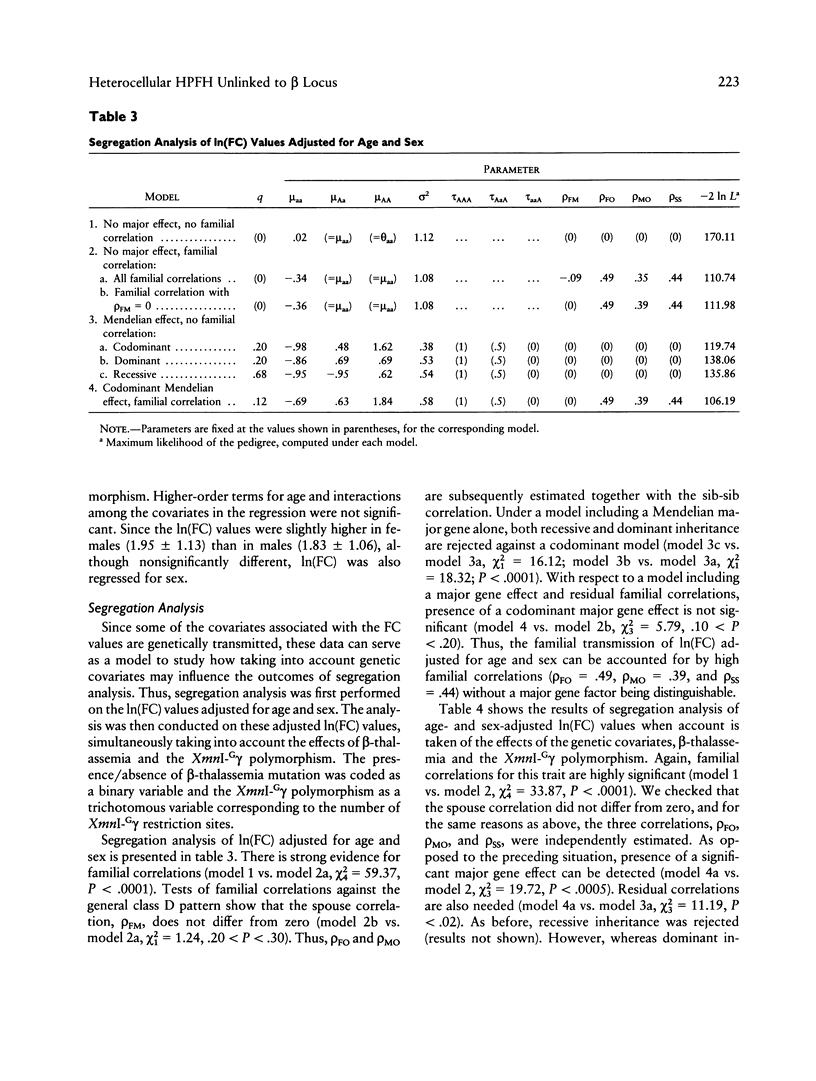
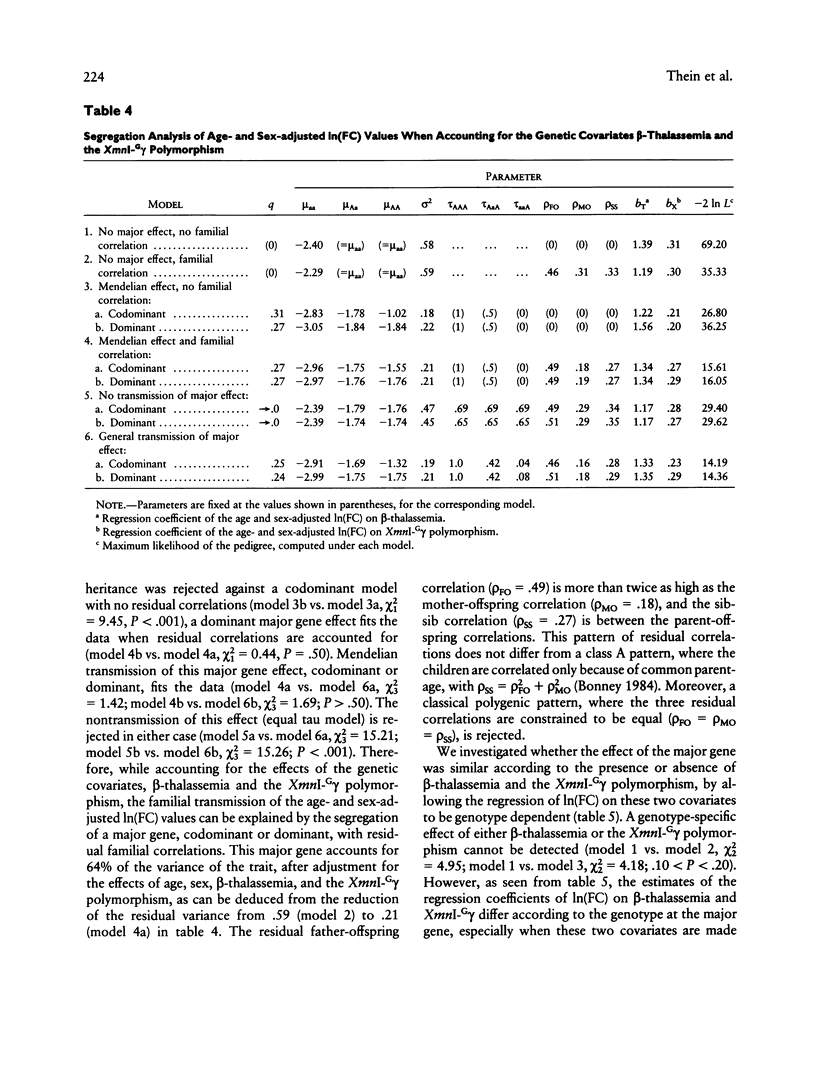
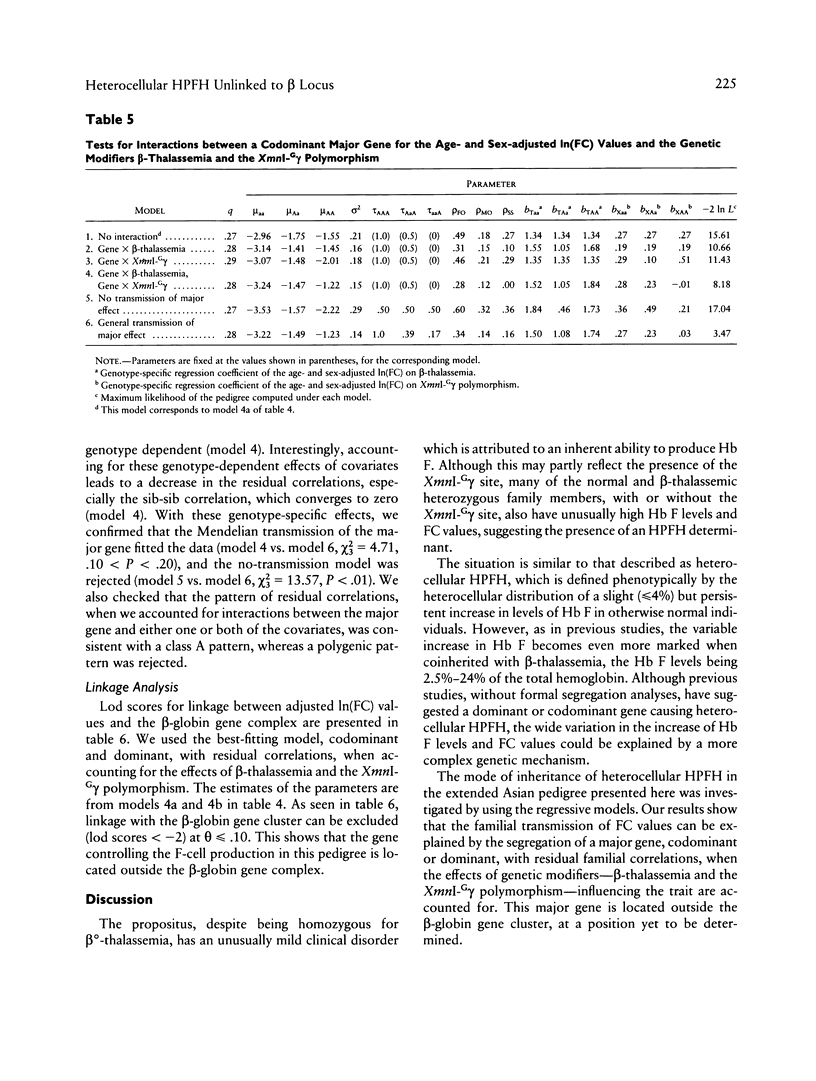
"Heterocellular hereditary persistence of fetal hemoglobin" (HPFH) is the term used to describe the genetically determined persistence of fetal hemoglobin (Hb F) production into adult life, in the absence of any related hematological disorder. Whereas some forms are caused by mutations in the beta-globin gene cluster on chromosome 11, others segregate independently. While the latter are of particular interest with respect to the regulation of globin gene switching, it has not been possible to determine their chromosomal location, mainly because their mode of inheritance is not clear, but also because several other factors are known to modify Hb F production. We have examined a large Asian Indian pedigree which includes individuals with heterocellular HPFH associated with beta-thalassemia and/or alpha-thalassemia. Segregation analysis was conducted on the HPFH trait FC, defined to be the percentage of Hb F-containing cells (F-cells), using the class D regressive model. Our results provide evidence for the presence of a major gene, dominant or codominant, which controls the FC values with residual familial correlations. The major gene was detected when the effects of genetic modifiers, notably beta-thalassemia and the XmnI-G gamma polymorphism, are accounted for in the analysis. Linkage with the beta-globin gene cluster is excluded. The transmission of the FC values in this pedigree is informative enough to allow detection of linkage with an appropriate marker(s). The analytical approach outlined in this study, using simple regression to allow for genetic modifiers and thus allowing the mode of inheritance of a trait to be dissected out, may be useful as a model for segregation and linkage analyses of other complex phenotypes.
Full text
PDF

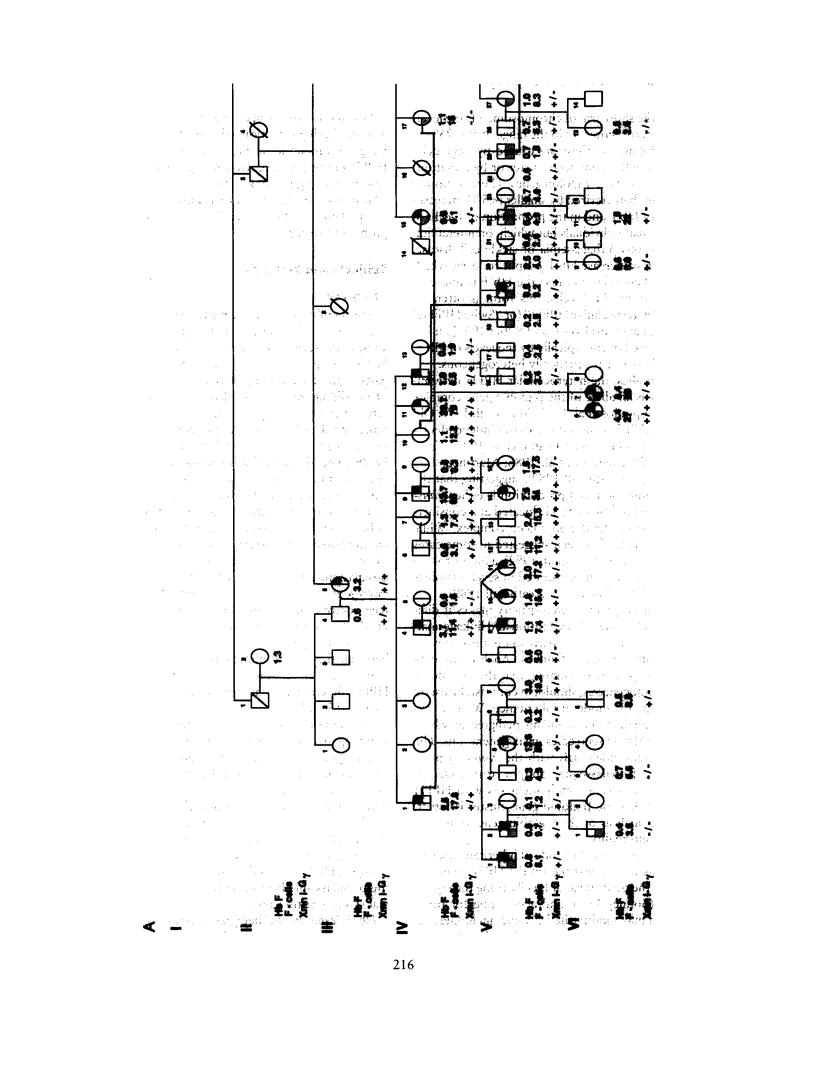
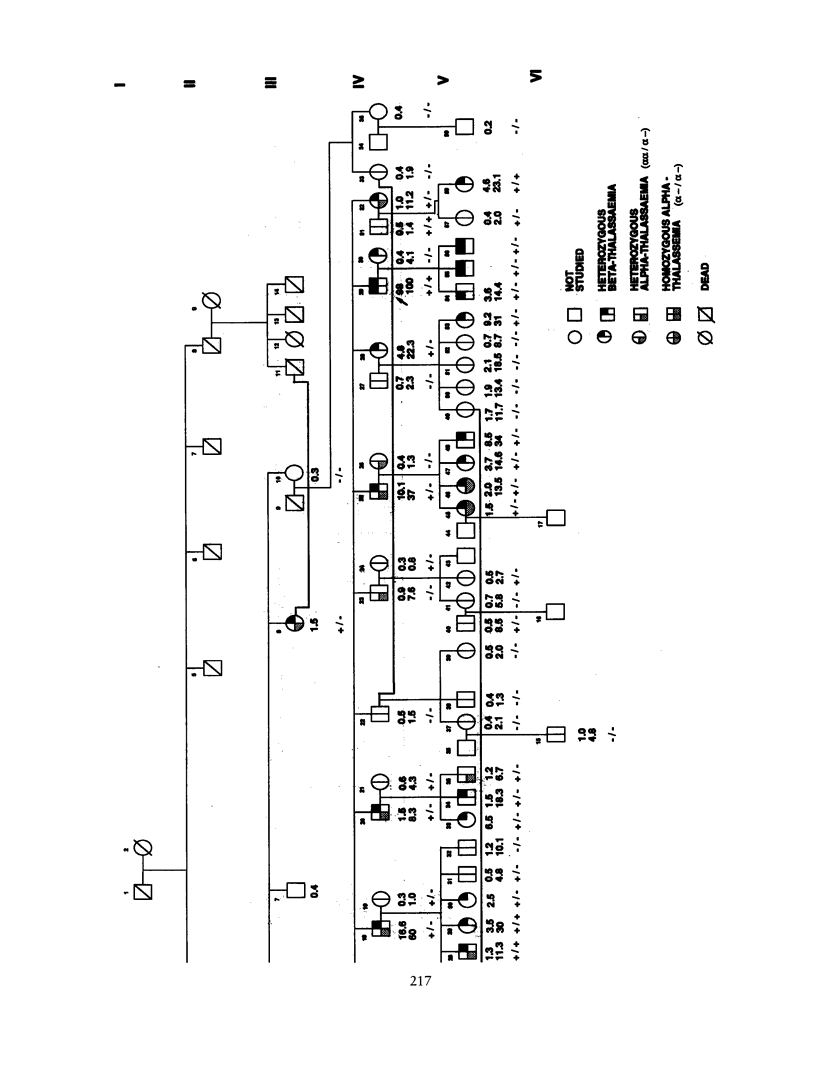
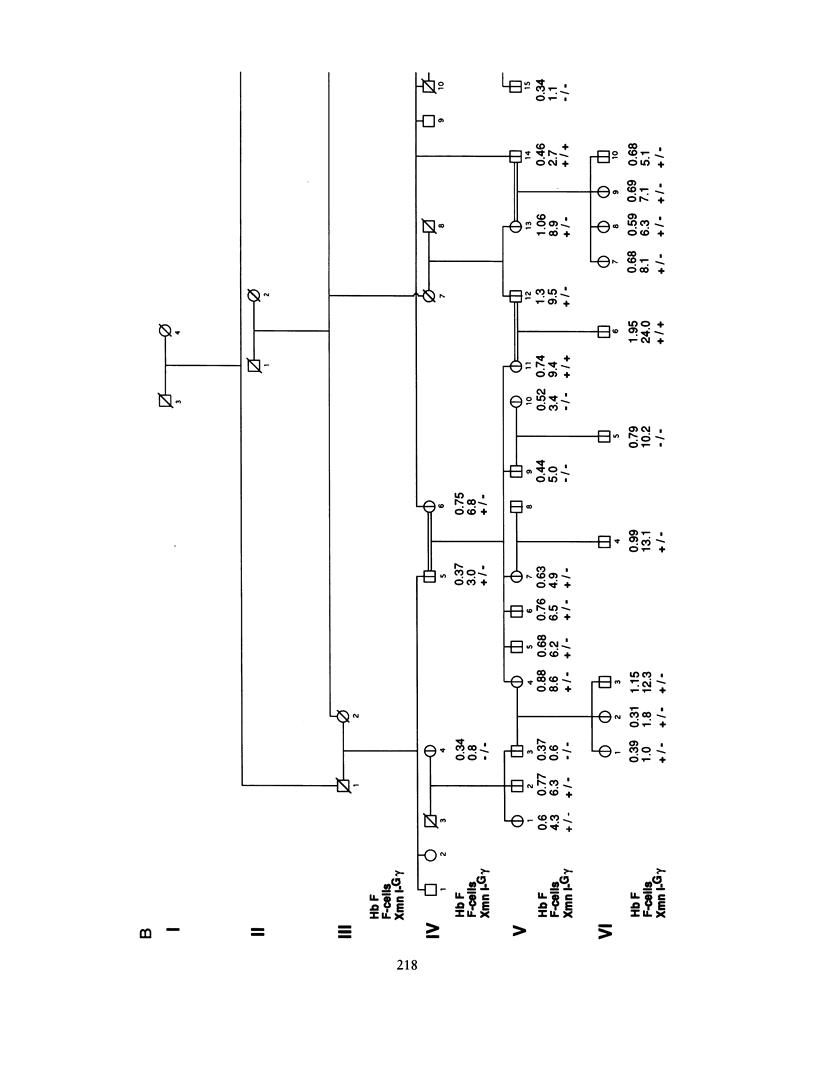
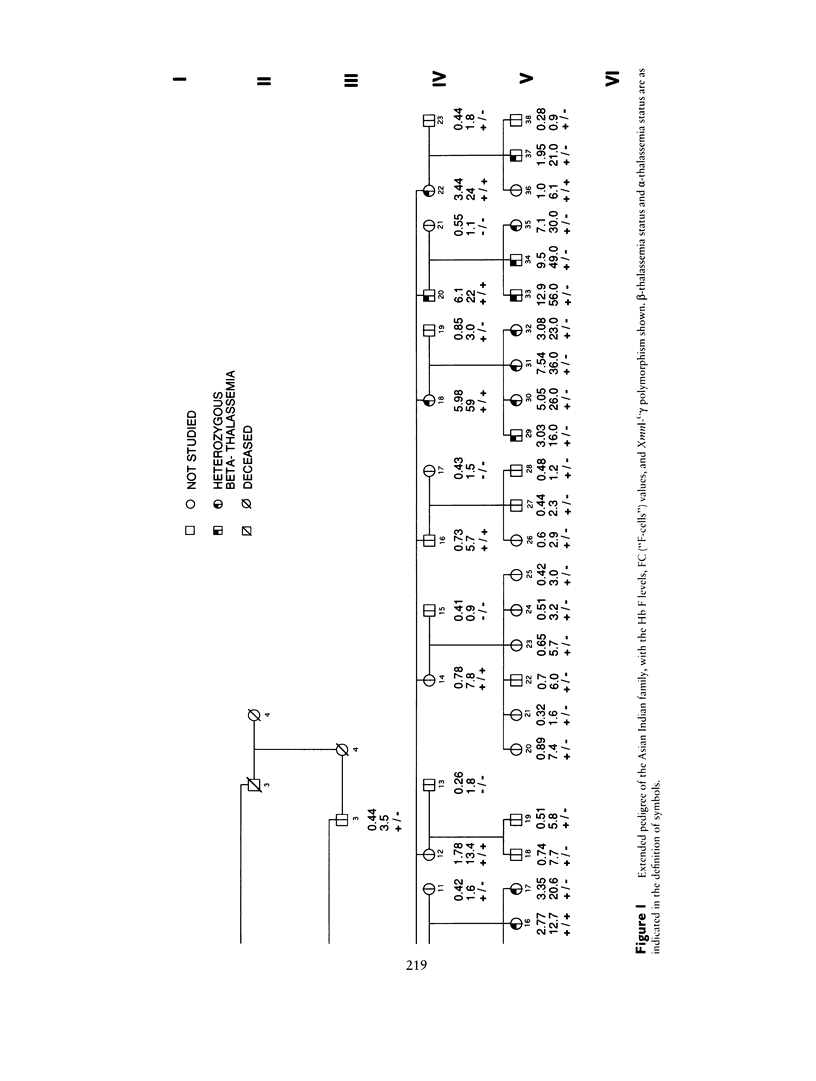




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altay C., Huisman T. H., Schroeder W. A. Another form of the hereditary persistence of fetal hemoglobin (the Atlanta type)? Hemoglobin. 1976;1(2):125–133. doi: 10.3109/03630267608991675. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- Bonney G. E., Lathrop G. M., Lalouel J. M. Combined linkage and segregation analysis using regressive models. Am J Hum Genet. 1988 Jul;43(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- Bonney G. E. On the statistical determination of major gene mechanisms in continuous human traits: regressive models. Am J Med Genet. 1984 Aug;18(4):731–749. doi: 10.1002/ajmg.1320180420. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Belding T. K., Margolet L., Noyes A. N. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science. 1975 Apr 25;188(4186):361–363. doi: 10.1126/science.804182. [DOI] [PubMed] [Google Scholar]
- Craig J. E., Sheerin S. M., Barnetson R., Thein S. L. The molecular basis of HPFH in a British family identified by heteroduplex formation. Br J Haematol. 1993 May;84(1):106–110. doi: 10.1111/j.1365-2141.1993.tb03032.x. [DOI] [PubMed] [Google Scholar]
- Demenais F. M., Bonney G. E. Equivalence of the mixed and regressive models for genetic analysis. I. Continuous traits. Genet Epidemiol. 1989;6(5):597–617. doi: 10.1002/gepi.1370060505. [DOI] [PubMed] [Google Scholar]
- Demenais F. M., Murigande C., Bonney G. E. Search for faster methods of fitting the regressive models to quantitative traits. Genet Epidemiol. 1990;7(5):319–334. doi: 10.1002/gepi.1370070503. [DOI] [PubMed] [Google Scholar]
- Demenais F., Lathrop M., Lalouel J. M. Robustness and power of the unified model in the analysis of quantitative measurements. Am J Hum Genet. 1986 Feb;38(2):228–234. [PMC free article] [PubMed] [Google Scholar]
- Dover G. J., Smith K. D., Chang Y. C., Purvis S., Mays A., Meyers D. A., Sheils C., Serjeant G. Fetal hemoglobin levels in sickle cell disease and normal individuals are partially controlled by an X-linked gene located at Xp22.2. Blood. 1992 Aug 1;80(3):816–824. [PubMed] [Google Scholar]
- Elston R. C., Stewart J. A general model for the genetic analysis of pedigree data. Hum Hered. 1971;21(6):523–542. doi: 10.1159/000152448. [DOI] [PubMed] [Google Scholar]
- Giampaolo A., Mavilio F., Sposi N. M., Carè A., Massa A., Cianetti L., Petrini M., Russo R., Cappellini M. D., Marinucci M. Heterocellular hereditary persistence of fetal hemoglobin (HPFH). Molecular mechanisms of abnormal gamma-gene expression in association with beta thalassemia and linkage relationship with the beta-globin gene cluster. Hum Genet. 1984;66(2-3):151–156. doi: 10.1007/BF00286590. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Bregni M., Cappellini M. D., Fiorelli G., Taramelli R., Giglioni B., Comi P., Ottolenghi S. A gene controlling fetal hemoglobin expression in adults is not linked to the non-alpha globin cluster. EMBO J. 1983;2(6):921–925. doi: 10.1002/j.1460-2075.1983.tb01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H. DNA sequence variation associated with elevated fetal G gamma globin production. Blood. 1985 Oct;66(4):783–787. [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L., Weatherall D. J., Ponder B. A. DNA "fingerprints" and segregation analysis of multiple markers in human pedigrees. Am J Hum Genet. 1986 Jul;39(1):11–24. [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTI H. R., BUETLER R. [Hemoglobin F and hemoglobin A2 increase in the Swiss population]. Acta Haematol. 1961;26:65–74. doi: 10.1159/000206642. [DOI] [PubMed] [Google Scholar]
- Martinez G., Novelletto A., Di Rienzo A., Felicetti L., Colombo B. A case of hereditary persistence of fetal hemoglobin caused by a gene not linked to the beta-globin cluster. Hum Genet. 1989 Jul;82(4):335–337. doi: 10.1007/BF00273993. [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Kaneto Y., Kawai H., Ohchi H., Niki S., Hasegawa K., Shirakami A., Yamano T. X-linked dominant control of F-cells in normal adult life: characterization of the Swiss type as hereditary persistence of fetal hemoglobin regulated dominantly by gene(s) on X chromosome. Blood. 1988 Dec;72(6):1854–1860. [PubMed] [Google Scholar]
- Old J. M., Ayyub H., Wood W. G., Clegg J. B., Weatherall D. J. Linkage analysis of nondeletion hereditary persistence of fetal hemoglobin. Science. 1982 Feb 19;215(4535):981–982. doi: 10.1126/science.6186021. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Rutland P. C., Pembrey M. E., Davies T. The estimation of fetal haemoglobin in healthy adults by radioimmunoassay. Br J Haematol. 1983 Apr;53(4):673–682. doi: 10.1111/j.1365-2141.1983.tb07319.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sampietro M., Thein S. L., Contreras M., Pazmany L. Variation of HbF and F-cell number with the G-gamma Xmn I (C-T) polymorphism in normal individuals. Blood. 1992 Feb 1;79(3):832–833. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Wood W. G., Papayannopoulou T., Nute P. E. A new form of hereditary persistence of fetal hemoglobin in blacks and its association with sickle cell trait. Blood. 1975 Nov;46(5):683–692. [PubMed] [Google Scholar]
- Thein S. L., Hinton J. A simple and rapid method of direct sequencing using Dynabeads. Br J Haematol. 1991 Sep;79(1):113–115. doi: 10.1111/j.1365-2141.1991.tb08016.x. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Stamatoyannopoulos G., Lim G., Nute P. E. F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F. Blood. 1975 Nov;46(5):671–682. [PubMed] [Google Scholar]
- Zago M. A., Wood W. G., Clegg J. B., Weatherall D. J., O'Sullivan M., Gunson H. Genetic control of F cells in human adults. Blood. 1979 May;53(5):977–986. [PubMed] [Google Scholar]


