Abstract
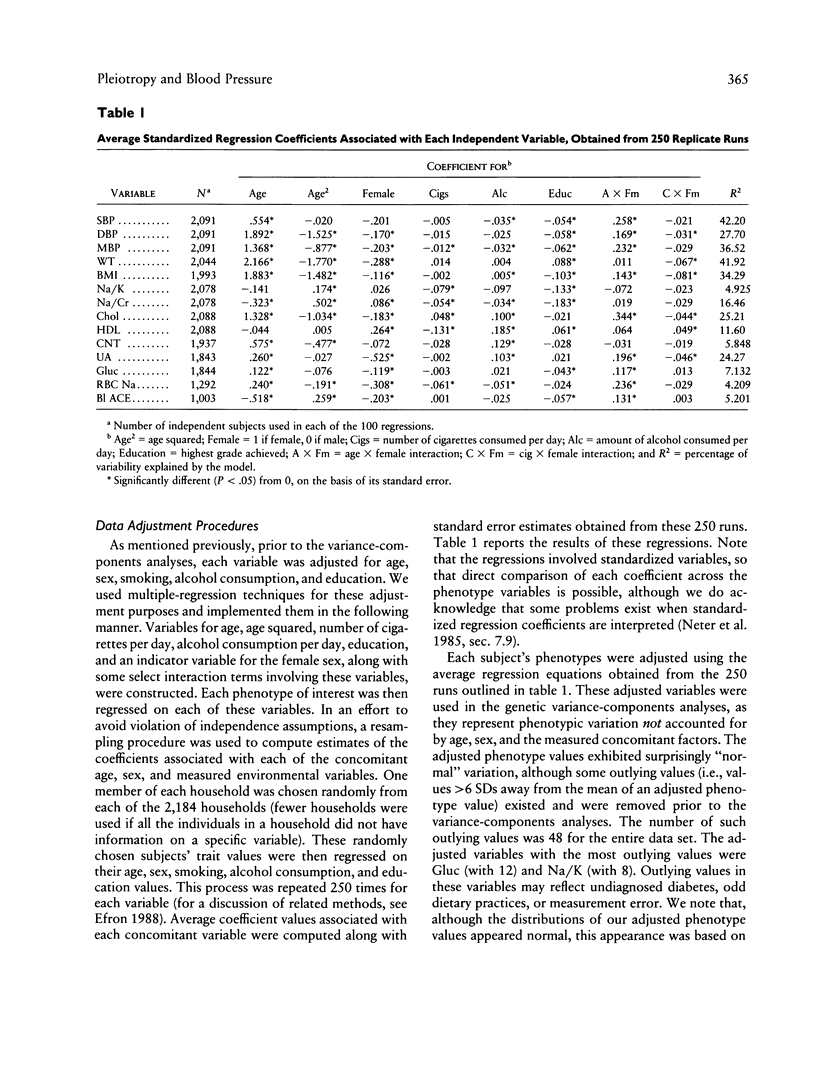
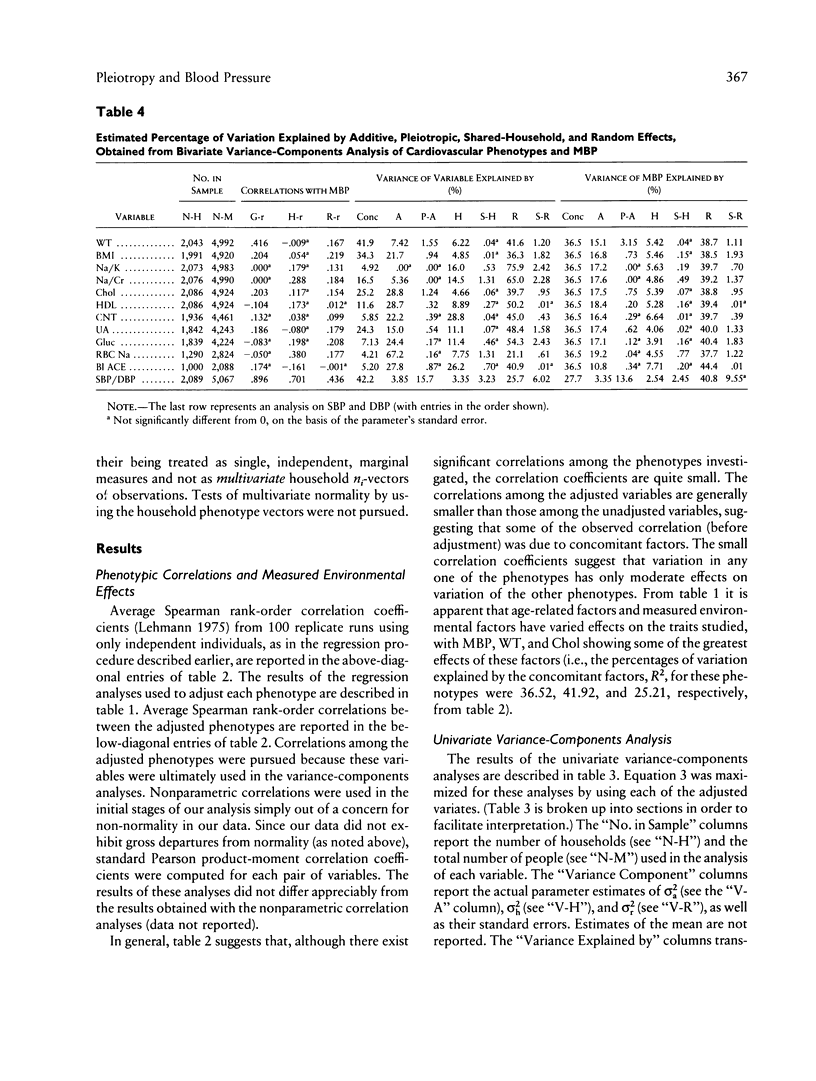
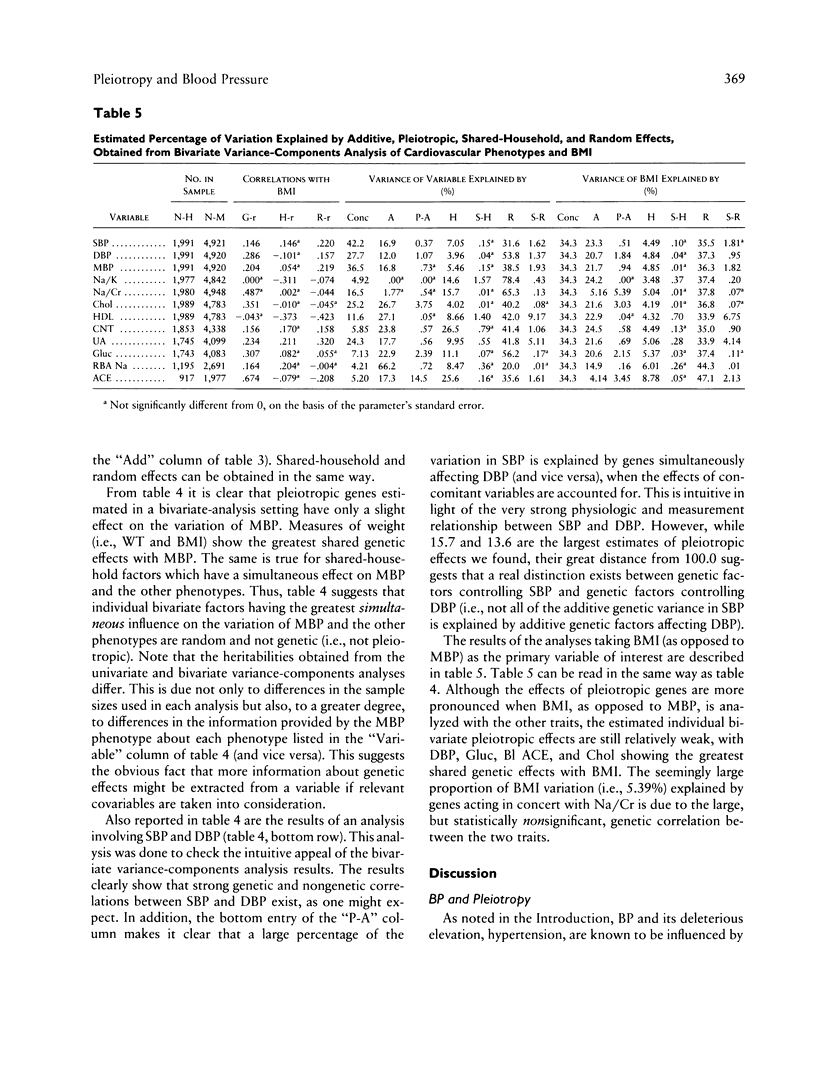
Blood pressure (BP), body-mass index (BMI), and quantitative phenotypes thought to influence BP (e.g., lithium-sodium countertransport activity) were studied in 2,184 households comprising 5,376 people in Gubbio, Italy. Variance-components models were used to partition the variation of these phenotypes into components characterizing the effects of age-related, measured environmental, additive genetic, pleiotropic, unmeasured shared-household, and individual-specific (or random) factors. The goal of the investigation was to estimate the contribution of pleiotropy to variation in BP and BMI in population-based samples. Although our results suggest that numerous significant bivariate genetic correlations exist between BP and some of the traits investigated, they ultimately lead us to reject a prominent role for any individual bivariate pleiotropic system influencing the natural variation of BP. However, because we found evidence that many traits enter into small-impact pleiotropic relationships with BP, we cannot rule out the possibility that pleiotropic genes, when considered collectively, may contribute to BP variation at the population level. Similar results were obtained when BMI was taken as the primary variable of interest. We argue that the small but significant portion of BP variation explained by individual genes displaying bivariate pleiotropic effects is intuitive, in light of the relatively low heritabilities associated with quantitative cardiovascular phenotypes and the low phenotypic correlations between BP, BMI, and many other physiologically linked measures of cardiovascular function. Our results not only bear directly on both the nature of the multifactorial determinants of BP and the maintenance of BP variation in the population at large, but also emphasize the utility of variance-components models in epidemiologic and population genetics research. We discuss the implications of our results for genetic epidemiologists and medical researchers studying hypertension, as well as the limitations of our study and areas for future research.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astemborski J. A., Beaty T. H., Cohen B. H. Variance components analysis of forced expiration in families. Am J Med Genet. 1985 Aug;21(4):741–753. doi: 10.1002/ajmg.1320210417. [DOI] [PubMed] [Google Scholar]
- Beaty T. H., Self S. G., Liang K. Y., Connolly M. A., Chase G. A., Kwiterovich P. O. Use of robust variance components models to analyse triglyceride data in families. Ann Hum Genet. 1985 Oct;49(Pt 4):315–328. doi: 10.1111/j.1469-1809.1985.tb01707.x. [DOI] [PubMed] [Google Scholar]
- Blangero J., Konigsberg L. W. Multivariate segregation analysis using the mixed model. Genet Epidemiol. 1991;8(5):299–316. doi: 10.1002/gepi.1370080503. [DOI] [PubMed] [Google Scholar]
- Carey G. Inference about genetic correlations. Behav Genet. 1988 May;18(3):329–338. doi: 10.1007/BF01260933. [DOI] [PubMed] [Google Scholar]
- Haffner S. M., Ferrannini E., Hazuda H. P., Stern M. P. Clustering of cardiovascular risk factors in confirmed prehypertensive individuals. Hypertension. 1992 Jul;20(1):38–45. doi: 10.1161/01.hyp.20.1.38. [DOI] [PubMed] [Google Scholar]
- Hanis C. L., Sing C. F., Clarke W. R., Schrott H. G. Multivariate models for human genetic analysis: aggregation, coaggregation, and tracking of systolic blood pressure and weight. Am J Hum Genet. 1983 Nov;35(6):1196–1210. [PMC free article] [PubMed] [Google Scholar]
- Hopper J. L., Mathews J. D. Extensions to multivariate normal models for pedigree analysis. II. Modeling the effect of shared environment in the analysis of variation in blood lead levels. Am J Epidemiol. 1983 Mar;117(3):344–355. doi: 10.1093/oxfordjournals.aje.a113547. [DOI] [PubMed] [Google Scholar]
- Jeunemaitre X., Soubrier F., Kotelevtsev Y. V., Lifton R. P., Williams C. S., Charru A., Hunt S. C., Hopkins P. N., Williams R. R., Lalouel J. M. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992 Oct 2;71(1):169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- Julius S., Pascual A. V., London R. Role of parasympathetic inhibition in the hyperkinetic type of borderline hypertension. Circulation. 1971 Sep;44(3):413–418. doi: 10.1161/01.cir.44.3.413. [DOI] [PubMed] [Google Scholar]
- Lange K., Boehnke M. Extensions to pedigree analysis. IV. Covariance components models for multivariate traits. Am J Med Genet. 1983 Mar;14(3):513–524. doi: 10.1002/ajmg.1320140315. [DOI] [PubMed] [Google Scholar]
- Laurenzi M., Cirillo M., Trevisan M. The Gubbio data. Epidemiology and pathophysiology. Gubbio Study Research Group. Clin Exp Hypertens A. 1992;14(1-2):261–269. doi: 10.3109/10641969209036187. [DOI] [PubMed] [Google Scholar]
- Laurenzi M., Trevisan M. Sodium-lithium countertransport and blood pressure: the Gubbio Population Study. Hypertension. 1989 May;13(5):408–415. doi: 10.1161/01.hyp.13.5.408. [DOI] [PubMed] [Google Scholar]
- Moll P. P., Burns T. L., Lauer R. M. The genetic and environmental sources of body mass index variability: the Muscatine Ponderosity Family Study. Am J Hum Genet. 1991 Dec;49(6):1243–1255. [PMC free article] [PubMed] [Google Scholar]
- Moll P. P., Powsner R., Sing C. F. Analysis of genetic and environmental sources of variation in serum cholesterol in Tecumseh, Michigan. V. Variance components estimated from pedigrees. Ann Hum Genet. 1979 Jan;42(3):343–354. doi: 10.1111/j.1469-1809.1979.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Pérusse L., Rice T., Bouchard C., Vogler G. P., Rao D. C. Cardiovascular risk factors in a French-Canadian population: resolution of genetic and familial environmental effects on blood pressure by using extensive information on environmental correlates. Am J Hum Genet. 1989 Aug;45(2):240–251. [PMC free article] [PubMed] [Google Scholar]
- Schork N. J., Schork M. A. The relative efficiency and power of small-pedigree studies of the heritability of a quantitative trait. Hum Hered. 1993 Jan-Feb;43(1):1–11. doi: 10.1159/000154106. [DOI] [PubMed] [Google Scholar]
- Schork N. J., Weder A. B., Schork M. A., Bassett D. R., Julius S. Disease entities, mixed multi-normal distributions, and the role of the hyperkinetic state in the pathogenesis of hypertension. Stat Med. 1990 Mar;9(3):301–314. doi: 10.1002/sim.4780090313. [DOI] [PubMed] [Google Scholar]
- Thompson E. A., Shaw R. G. Estimating polygenic models for multivariate data on large pedigrees. Genetics. 1992 Aug;131(4):971–978. doi: 10.1093/genetics/131.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. R., Hunt S. C., Hasstedt S. J., Hopkins P. N., Wu L. L., Berry T. D., Stults B. M., Barlow G. K., Schumacher M. C., Lifton R. P. Multigenic human hypertension: evidence for subtypes and hope for haplotypes. J Hypertens Suppl. 1990 Dec;8(7):S39–S46. [PubMed] [Google Scholar]
- Williams R. R., Hunt S. C., Hopkins P. N., Stults B. M., Wu L. L., Hasstedt S. J., Barlow G. K., Stephenson S. H., Lalouel J. M., Kuida H. Familial dyslipidemic hypertension. Evidence from 58 Utah families for a syndrome present in approximately 12% of patients with essential hypertension. JAMA. 1988 Jun 24;259(24):3579–3586. doi: 10.1001/jama.259.24.3579. [DOI] [PubMed] [Google Scholar]


