Abstract
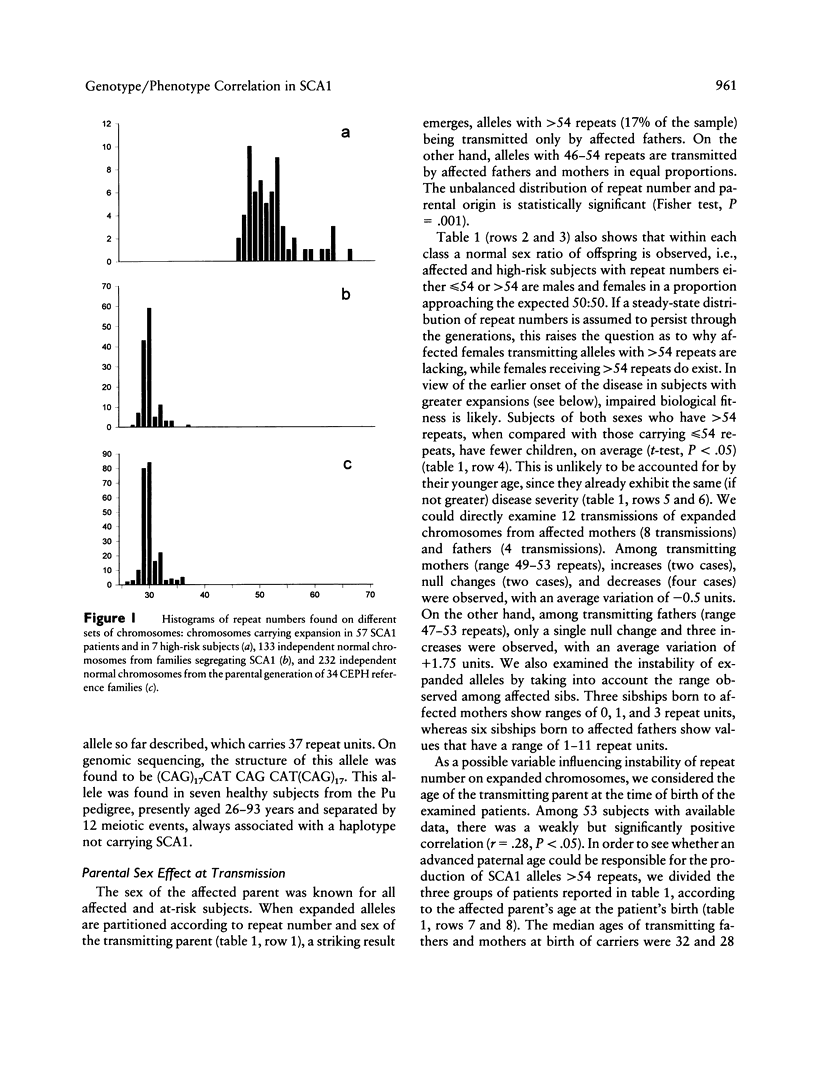
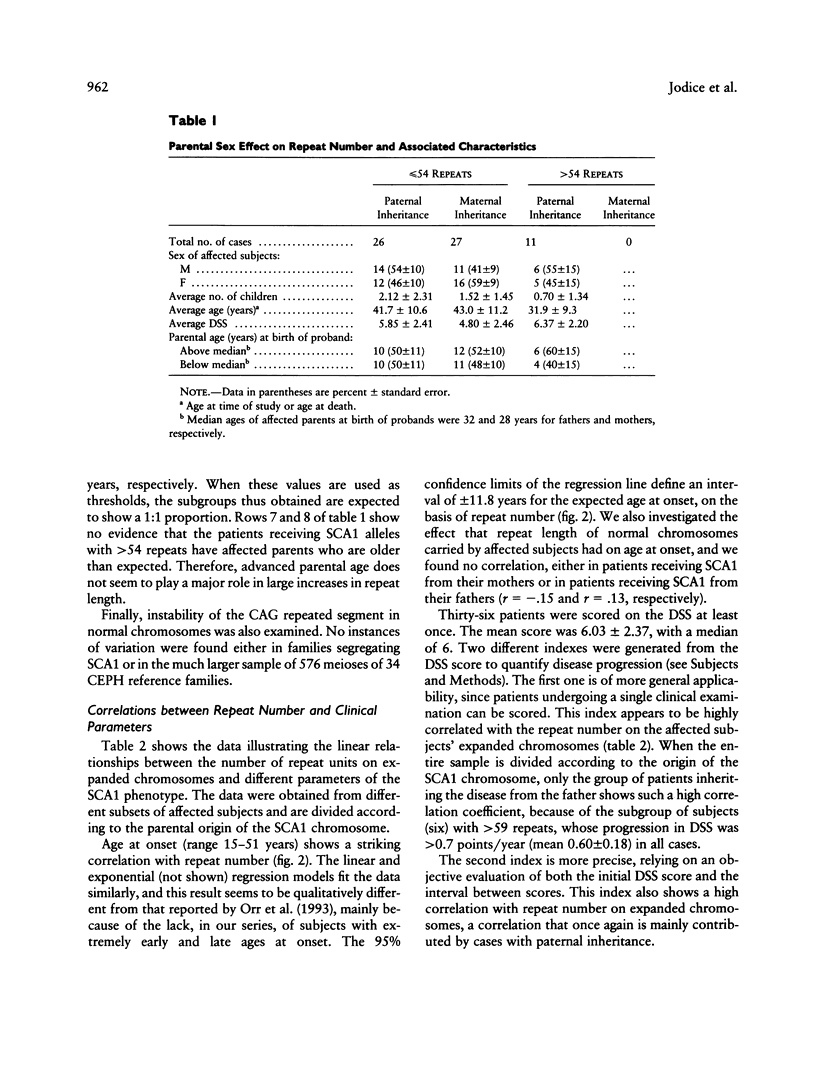
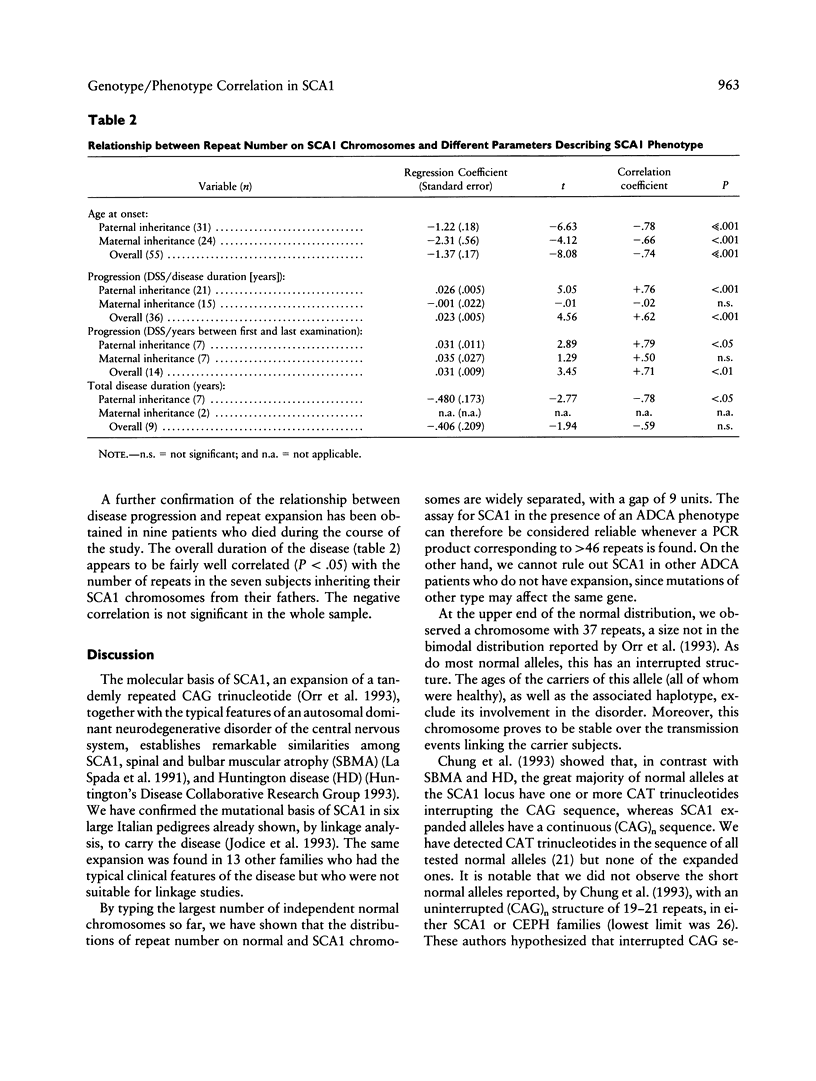
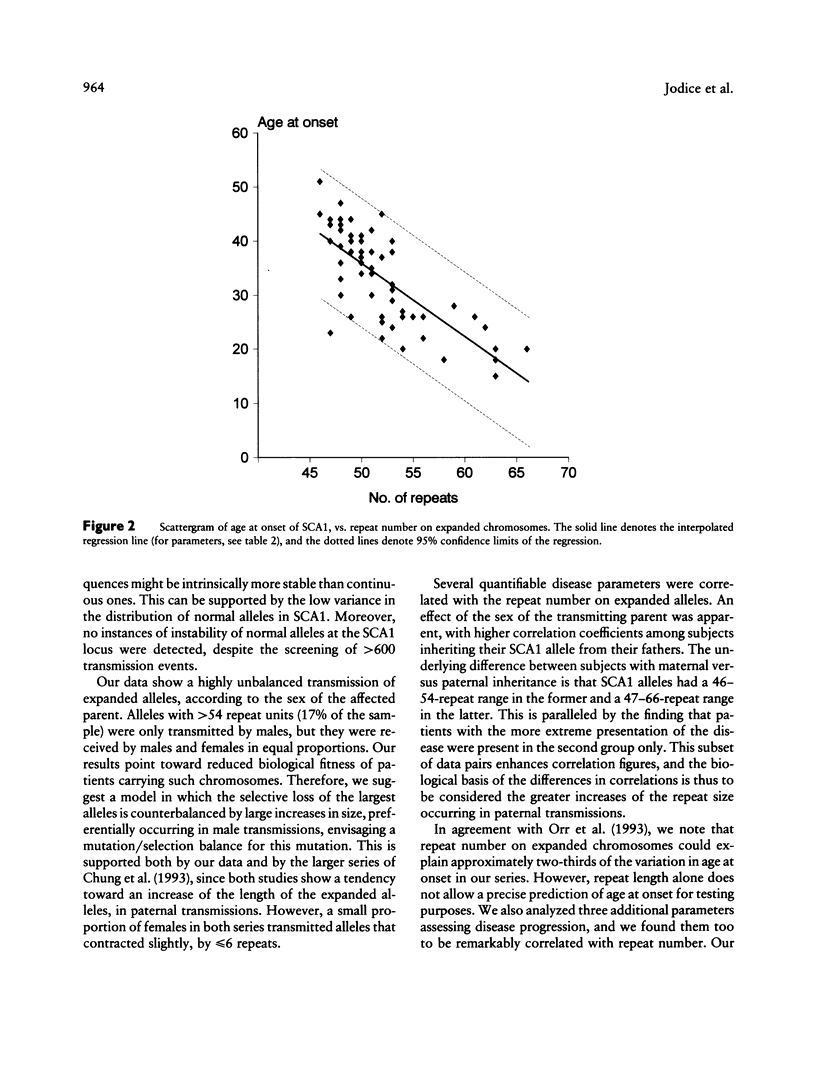
Trinucleotide repeat expansion has been found in 64 subjects from 19 families: 57 patients with SCA1 and 7 subjects predicted, by haplotype analysis, to carry the mutation. Comparison with a large set of normal chromosomes shows two distinct distributions, with a much wider variation among expanded chromosomes. The sex of transmitting parent plays a major role in the size distribution of expanded alleles, those with > 54 repeats being transmitted by affected fathers exclusively. Our data suggest that alleles with > 54 repeats have a reduced chance of survival; these appear to be replaced in each generation by further expansion of alleles in the low- to medium-expanded repeat range, preferentially in male transmissions. Detailed clinical follow-up of a subset of our patients demonstrates significant relationships between increasing repeat number on expanded chromosomes and earlier age at onset, faster progression of the disease, and earlier age at death.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung M. Y., Ranum L. P., Duvick L. A., Servadio A., Zoghbi H. Y., Orr H. T. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet. 1993 Nov;5(3):254–258. doi: 10.1038/ng1193-254. [DOI] [PubMed] [Google Scholar]
- Gispert S., Twells R., Orozco G., Brice A., Weber J., Heredero L., Scheufler K., Riley B., Allotey R., Nothers C. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet. 1993 Jul;4(3):295–299. doi: 10.1038/ng0793-295. [DOI] [PubMed] [Google Scholar]
- Green H. Human genetic diseases due to codon reiteration: relationship to an evolutionary mechanism. Cell. 1993 Sep 24;74(6):955–956. doi: 10.1016/0092-8674(93)90718-6. [DOI] [PubMed] [Google Scholar]
- Harding A. E. Genetic aspects of autosomal dominant late onset cerebellar ataxia. J Med Genet. 1981 Dec;18(6):436–441. doi: 10.1136/jmg.18.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodice C., Frontali M., Persichetti F., Novelletto A., Pandolfo M., Spadaro M., Giunti P., Schinaia G., Lulli P., Malaspina P. The gene for spinal cerebellar ataxia 1 (SCA1) is flanked by two closely linked highly polymorphic microsatellite loci. Hum Mol Genet. 1993 Sep;2(9):1383–1387. doi: 10.1093/hmg/2.9.1383. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski T. J., Jr, Orr H. T., Banfi S., McCall A. E., Jodice C., Persichetti F., Novelletto A., LeBorgne-DeMarquoy F., Duvick L. A., Frontali M. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps centromeric to D6S89 and shows no recombination, in nine large kindreds, with a dinucleotide repeat at the AM10 locus. Am J Hum Genet. 1993 Aug;53(2):391–400. [PMC free article] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Morton N. E., Lalouel J. M., Jackson J. F., Currier R. D., Yee S. Linkage studies in spinocerebellar ataxia (SCA). Am J Med Genet. 1980;6(3):251–257. doi: 10.1002/ajmg.1320060309. [DOI] [PubMed] [Google Scholar]
- Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989 Nov 11;17(21):8889–8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Ranum L. P., Duvick L. A., Rich S. S., Schut L. J., Litt M., Orr H. T. Localization of the autosomal dominant HLA-linked spinocerebellar ataxia (SCA1) locus, in two kindreds, within an 8-cM subregion of chromosome 6p. Am J Hum Genet. 1991 Jul;49(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- Shrimpton A. E., Davidson R., MacDonald N., Brock D. J. Presymptomatic testing for autosomal dominant spinocerebellar ataxia type 1. J Med Genet. 1993 Jul;30(7):616–617. doi: 10.1136/jmg.30.7.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro M., Giunti P., Lulli P., Frontali M., Jodice C., Cappellacci S., Morellini M., Persichetti F., Trabace S., Anastasi R. HLA-linked spinocerebellar ataxia: a clinical and genetic study of large Italian kindreds. Acta Neurol Scand. 1992 Apr;85(4):257–265. doi: 10.1111/j.1600-0404.1992.tb04041.x. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Nishizawa M., Tanaka H., Kawashima S., Sakamoto H., Karube Y., Shimazaki H., Soutome M., Endo K., Ohta S. The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet. 1993 Jul;4(3):300–304. doi: 10.1038/ng0793-300. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Jodice C., Sandkuijl L. A., Kwiatkowski T. J., Jr, McCall A. E., Huntoon S. A., Lulli P., Spadaro M., Litt M., Cann H. M. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps telomeric to the HLA complex and is closely linked to the D6S89 locus in three large kindreds. Am J Hum Genet. 1991 Jul;49(1):23–30. [PMC free article] [PubMed] [Google Scholar]


