Abstract
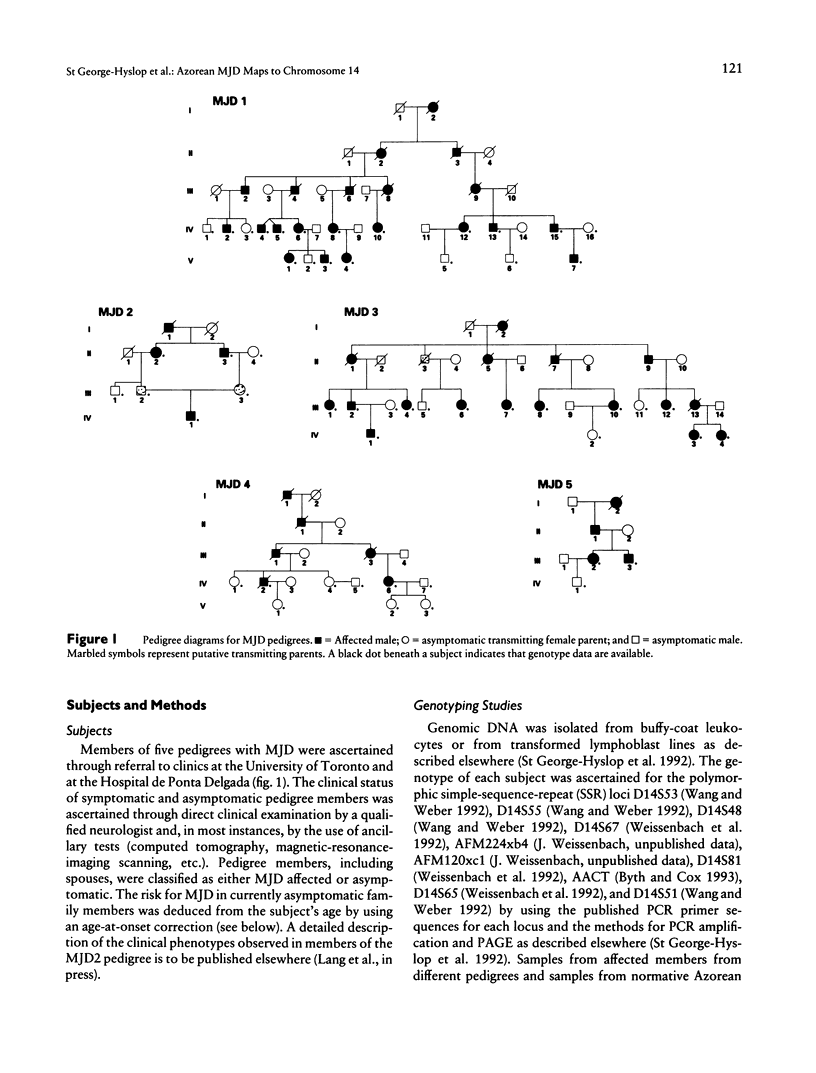
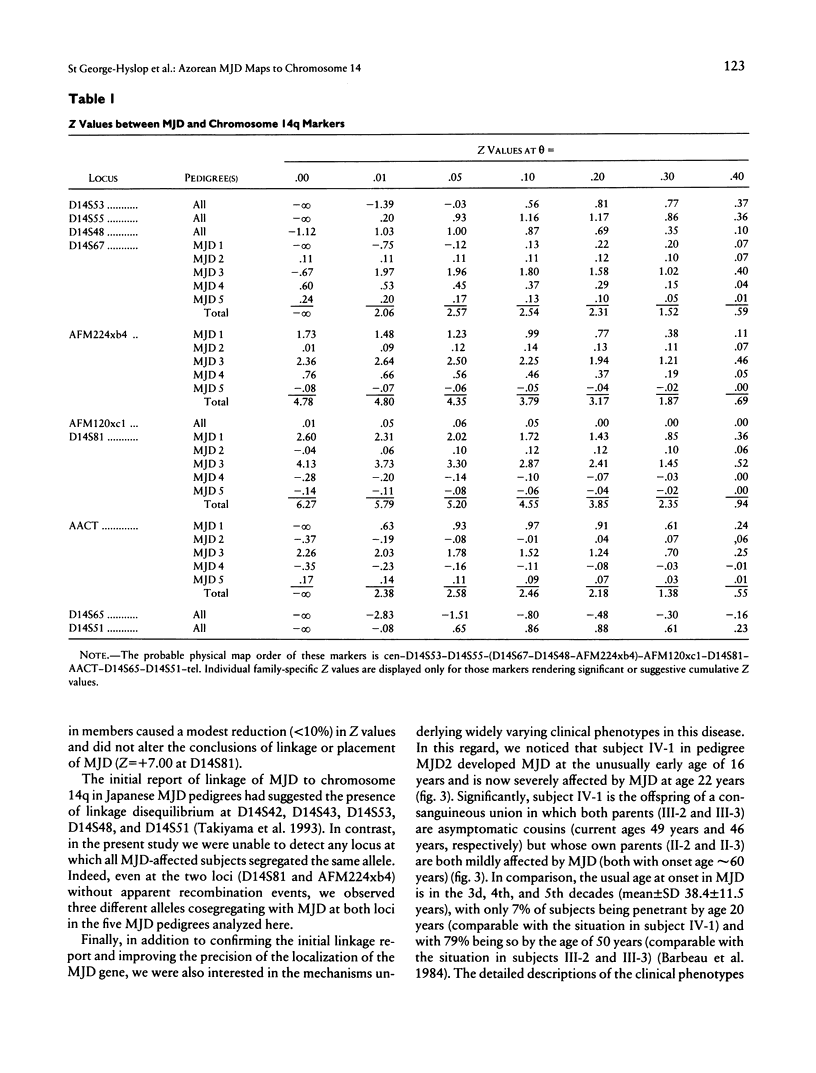
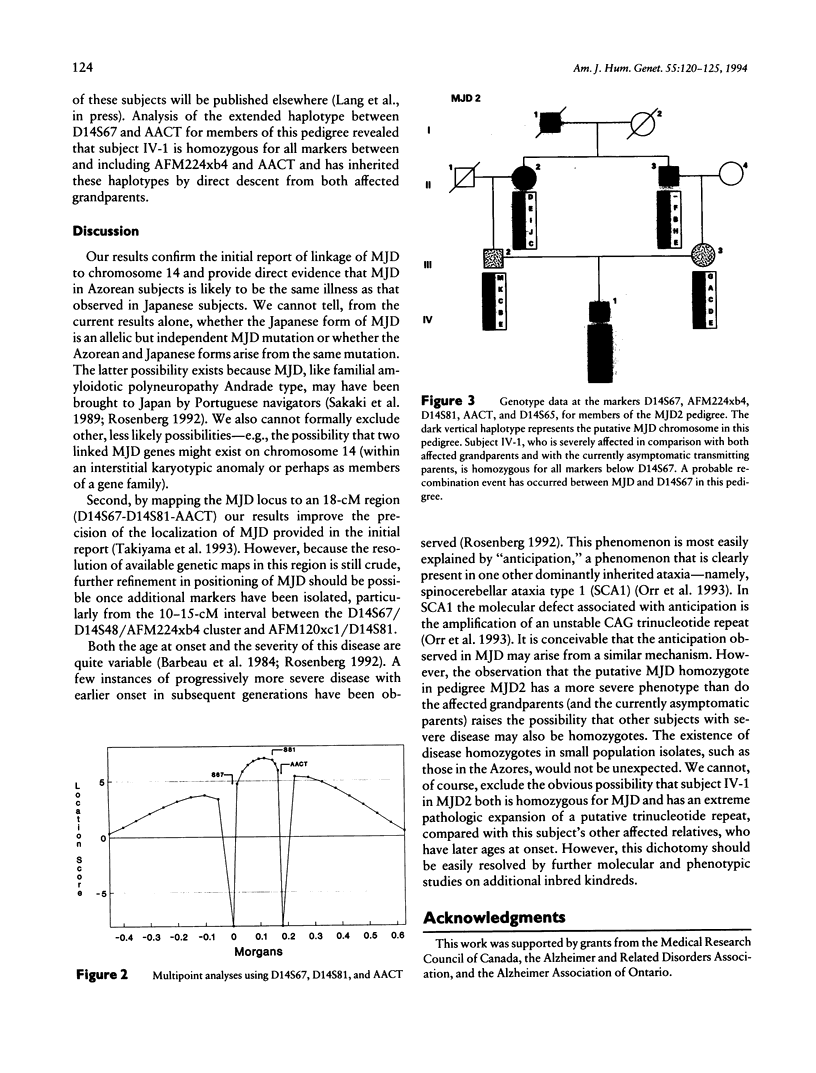
A locus for Machado-Joseph disease (MJD) has recently been mapped to a 30-cM region of chromosome 14q in five pedigrees of Japanese descent. MJD is a clinically pleomorphic neurodegenerative disease that was originally described in subjects of Azorean descent. In light of the nonallelic heterogeneity in other inherited spinocere-bellar ataxias, we were interested to determine if the MJD phenotype in Japanese and Azorean pedigrees arose from mutations at the same locus. We provide evidence that MJD in five pedigrees of Azorean descent is also linked to chromosome 14q in an 18-cM region between the markers D14S67 and AACT (multipoint lod score +7.00 near D14S81). We also report molecular evidence for homozy-gosity at the MJD locus in an MJD-affected subject with severe, early-onset symptoms. These observations confirm the initial report of linkage of MJD to chromosome 14; suggest that MJD in Japanese and Azorean subjects may represent allelic or identical mutations at the same locus; and provide one possible explanation (MJD gene dosage) for the observed phenotypic heterogeneity in this disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbeau A., Roy M., Cunha L., de Vincente A. N., Rosenberg R. N., Nyhan W. L., MacLeod P. L., Chazot G., Langston L. B., Dawson D. M. The natural history of Machado-Joseph disease. An analysis of 138 personally examined cases. Can J Neurol Sci. 1984 Nov;11(4 Suppl):510–525. doi: 10.1017/s0317167100034983. [DOI] [PubMed] [Google Scholar]
- Braverman M. S. An algorithm to improve the computational efficiency of genetic linkage analysis. Comput Biomed Res. 1985 Feb;18(1):24–36. doi: 10.1016/0010-4809(85)90004-7. [DOI] [PubMed] [Google Scholar]
- Byth B. C., Cox D. W. Two consecutive dinucleotide repeats constitute an informative marker at the alpha 1-antichymotrypsin (AACT) locus. Hum Mol Genet. 1993 Jul;2(7):1085–1085. doi: 10.1093/hmg/2.7.1085-a. [DOI] [PubMed] [Google Scholar]
- Carson W. J., Radvany J., Farrer L. A., Vincent D., Rosenberg R. N., MacLeod P. M., Rouleau G. A. The Machado-Joseph disease locus is different from the spinocerebellar ataxia locus (SCA1). Genomics. 1992 Jul;13(3):852–855. doi: 10.1016/0888-7543(92)90168-r. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Nakada T., Knight R. T., Friedland R. P. Autosomal dominant motor system degeneration in a black family. Ann Neurol. 1983 Nov;14(5):585–587. doi: 10.1002/ana.410140515. [DOI] [PubMed] [Google Scholar]
- Cunha L., Gonçalves A., Dinis M., Oliveira C., Ferro M., Vicente A., Roy M., Barbeau A. Dominantly inherited ataxias in Portugal. Can J Neurol Sci. 1988 Nov;15(4):397–401. [PubMed] [Google Scholar]
- Gispert S., Twells R., Orozco G., Brice A., Weber J., Heredero L., Scheufler K., Riley B., Allotey R., Nothers C. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet. 1993 Jul;4(3):295–299. doi: 10.1038/ng0793-295. [DOI] [PubMed] [Google Scholar]
- Jain S., Maheshwari M. C. Eight families with Joseph's disease in India. Neurology. 1990 Jan;40(1):128–131. doi: 10.1212/wnl.40.1.128. [DOI] [PubMed] [Google Scholar]
- Nakano K. K., Dawson D. M., Spence A. Machado disease. A hereditary ataxia in Portuguese emigrants to Massachusetts. Neurology. 1972 Jan;22(1):49–55. doi: 10.1212/wnl.22.1.49. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. N. Machado-Joseph disease: an autosomal dominant motor system degeneration. Mov Disord. 1992;7(3):193–203. doi: 10.1002/mds.870070302. [DOI] [PubMed] [Google Scholar]
- Sakai T., Ohta M., Ishino H. Joseph disease in a non-Portuguese family. Neurology. 1983 Jan;33(1):74–80. doi: 10.1212/wnl.33.1.74. [DOI] [PubMed] [Google Scholar]
- Sakaki Y., Yoshioka K., Tanahashi H., Furuya H., Sasaki H. Human transthyretin (prealbumin) gene and molecular genetics of familial amyloidotic polyneuropathy. Mol Biol Med. 1989 Apr;6(2):161–168. [PubMed] [Google Scholar]
- Sequeiros J., Coutinho P. Epidemiology and clinical aspects of Machado-Joseph disease. Adv Neurol. 1993;61:139–153. [PubMed] [Google Scholar]
- Silveira I., Manaia A., Melki J., Magariño C., Lunkes A., Hernandez A., Gispert S., Burlet P., Rozet J. M., Coutinho P. Machado-Joseph disease is genetically different from Holguin dominant ataxia (SCA2). Genomics. 1993 Sep;17(3):556–559. doi: 10.1006/geno.1993.1371. [DOI] [PubMed] [Google Scholar]
- Spinella G. M., Sheridan P. H. Research initiatives on Machado-Joseph disease: National Institute of Neurological Disorders and Stroke Workshop summary. Neurology. 1992 Oct;42(10):2048–2051. doi: 10.1212/wnl.42.10.2048. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P., Haines J., Rogaev E., Mortilla M., Vaula G., Pericak-Vance M., Foncin J. F., Montesi M., Bruni A., Sorbi S. Genetic evidence for a novel familial Alzheimer's disease locus on chromosome 14. Nat Genet. 1992 Dec;2(4):330–334. doi: 10.1038/ng1292-330. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Nishizawa M., Tanaka H., Kawashima S., Sakamoto H., Karube Y., Shimazaki H., Soutome M., Endo K., Ohta S. The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet. 1993 Jul;4(3):300–304. doi: 10.1038/ng0793-300. [DOI] [PubMed] [Google Scholar]
- Wang Z., Weber J. L. Continuous linkage map of human chromosome 14 short tandem repeat polymorphisms. Genomics. 1992 Jul;13(3):532–536. doi: 10.1016/0888-7543(92)90121-8. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]


