Abstract
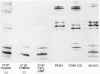
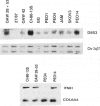
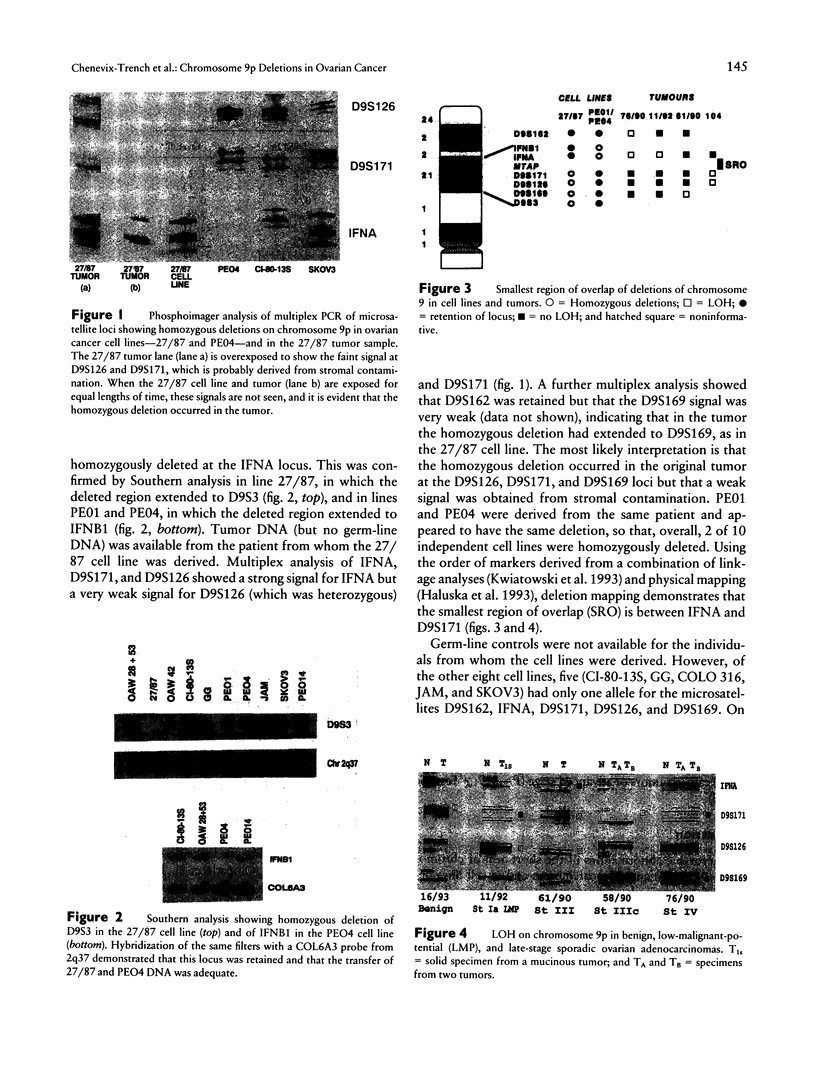
Rat ovarian surface epithelial cells transformed spontaneously in vitro have been found to have homozygous deletions of the interferon alpha (IFNA) gene. This suggests that inactivation of a tumor-suppressor gene in this region may be crucial for the development of ovarian cancer. We therefore used microsatellite markers and Southern analysis to examine the homologous region in humans--the short arm of chromosome 9--for deletions in sporadic ovarian adenocarcinomas and ovarian tumor cell lines. Loss of heterozygosity occurred in 34 (37%) of 91 informative sporadic tumors, including some benign, low-malignant-potential and early-stage tumors, suggesting that it is an early event in the development of ovarian adenocarcinoma. Furthermore, homozygous deletions on 9p were found in 2 of 10 independent cell lines. Deletion mapping of the tumors and lines indicates that the candidate suppressor gene inactivated as a consequence lies between D9S171 and the IFNA locus, a region that is also deleted in several other tumors and that contains the melanoma predisposition gene, MLM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello M. J., Moreno S., Rey J. A. Involvement of 9p in metastatic ovarian adenocarcinomas. Cancer Genet Cytogenet. 1990 Apr;45(2):223–229. doi: 10.1016/0165-4608(90)90086-p. [DOI] [PubMed] [Google Scholar]
- Bello M. J., Rey J. A. Chromosome aberrations in metastatic ovarian cancer: relationship with abnormalities in primary tumors. Int J Cancer. 1990 Jan 15;45(1):50–54. doi: 10.1002/ijc.2910450111. [DOI] [PubMed] [Google Scholar]
- Bertoncello I., Bradley T. R., Webber L. M., Hodgson G. S., Campbell J. J. Human tumour cell lines established using clonal agar culture. Aust J Exp Biol Med Sci. 1985 Apr;63(Pt 2):241–248. doi: 10.1038/icb.1985.27. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Center R., Lukeis R., Dietzsch E., Gillespie M., Garson O. M. Molecular deletion of 9p sequences in non-small cell lung cancer and malignant mesothelioma. Genes Chromosomes Cancer. 1993 May;7(1):47–53. doi: 10.1002/gcc.2870070108. [DOI] [PubMed] [Google Scholar]
- Chenevix-Trench G., Leary J., Kerr J., Michel J., Kefford R., Hurst T., Parsons P. G., Friedlander M., Khoo S. K. Frequent loss of heterozygosity on chromosome 18 in ovarian adenocarcinoma which does not always include the DCC locus. Oncogene. 1992 Jun;7(6):1059–1065. [PubMed] [Google Scholar]
- Chenevix-Trench G., Wicking C., Berkman J., Sharpe H., Hockey A., Haan E., Oley C., Ravine D., Turner A., Goldgar D. Further localization of the gene for nevoid basal cell carcinoma syndrome (NBCCS) in 15 Australasian families: linkage and loss of heterozygosity. Am J Hum Genet. 1993 Sep;53(3):760–767. [PMC free article] [PubMed] [Google Scholar]
- Cliby W., Ritland S., Hartmann L., Dodson M., Halling K. C., Keeney G., Podratz K. C., Jenkins R. B. Human epithelial ovarian cancer allelotype. Cancer Res. 1993 May 15;53(10 Suppl):2393–2398. [PubMed] [Google Scholar]
- Diaz M. O., Rubin C. M., Harden A., Ziemin S., Larson R. A., Le Beau M. M., Rowley J. D. Deletions of interferon genes in acute lymphoblastic leukemia. N Engl J Med. 1990 Jan 11;322(2):77–82. doi: 10.1056/NEJM199001113220202. [DOI] [PubMed] [Google Scholar]
- Eccles D. M., Russell S. E., Haites N. E., Atkinson R., Bell D. W., Gruber L., Hickey I., Kelly K., Kitchener H., Leonard R. Early loss of heterozygosity on 17q in ovarian cancer. The Abe Ovarian Cancer Genetics Group. Oncogene. 1992 Oct;7(10):2069–2072. [PubMed] [Google Scholar]
- Fathalla M. F. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971 Jul 17;2(7716):163–163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Foulkes W. D., Ragoussis J., Stamp G. W., Allan G. J., Trowsdale J. Frequent loss of heterozygosity on chromosome 6 in human ovarian carcinoma. Br J Cancer. 1993 Mar;67(3):551–559. doi: 10.1038/bjc.1993.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Taruscio D., Graw S. L., Buckler A. J., Ward D. C., Dracopoli N. C., Housman D. E. Genetic and physical map of the interferon region on chromosome 9p. Genomics. 1992 Sep;14(1):105–112. doi: 10.1016/s0888-7543(05)80290-3. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Söderkvist P., Marks J. R., Iglehart J. D., Cochran C., Barrett J. C., Wiseman R. W. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992 May 1;52(9):2624–2627. [PubMed] [Google Scholar]
- Gallion H. H., Powell D. E., Morrow J. K., Pieretti M., Case E., Turker M. S., DePriest P. D., Hunter J. E., van Nagell J. R., Jr Molecular genetic changes in human epithelial ovarian malignancies. Gynecol Oncol. 1992 Nov;47(2):137–142. doi: 10.1016/0090-8258(92)90096-2. [DOI] [PubMed] [Google Scholar]
- Godbout R., Miyakoshi J., Dobler K. D., Andison R., Matsuo K., Allalunis-Turner M. J., Takebe H., Day R. S., 3rd Lack of expression of tumor-suppressor genes in human malignant glioma cell lines. Oncogene. 1992 Sep;7(9):1879–1884. [PubMed] [Google Scholar]
- Godwin A. K., Testa J. R., Handel L. M., Liu Z., Vanderveer L. A., Tracey P. A., Hamilton T. C. Spontaneous transformation of rat ovarian surface epithelial cells: association with cytogenetic changes and implications of repeated ovulation in the etiology of ovarian cancer. J Natl Cancer Inst. 1992 Apr 15;84(8):592–601. doi: 10.1093/jnci/84.8.592. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kunzmann R., Hölzel F. Karyotype alterations in human ovarian carcinoma cells during long-term cultivation and nude mouse passage. Cancer Genet Cytogenet. 1987 Oct;28(2):201–212. doi: 10.1016/0165-4608(87)90206-8. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Armour J., Bale A. E., Fountain J. W., Goudie D., Haines J. L., Knowles M. A., Pilz A., Slaugenhaupt S., Povey S. Report and abstracts of the Second International Workshop on Human Chromosome 9 Mapping 1993. Cytogenet Cell Genet. 1993;64(2):93–121. doi: 10.1159/000133566. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Diaz M. O. Dinucleotide repeat polymorphism at the IFNA locus (9p22). Hum Mol Genet. 1992 Nov;1(8):658–658. doi: 10.1093/hmg/1.8.658-a. [DOI] [PubMed] [Google Scholar]
- Langdon S. P., Lawrie S. S., Hay F. G., Hawkes M. M., McDonald A., Hayward I. P., Schol D. J., Hilgers J., Leonard R. C., Smyth J. F. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988 Nov 1;48(21):6166–6172. [PubMed] [Google Scholar]
- Lee J. H., Kavanagh J. J., Wildrick D. M., Wharton J. T., Blick M. Frequent loss of heterozygosity on chromosomes 6q, 11, and 17 in human ovarian carcinomas. Cancer Res. 1990 May 1;50(9):2724–2728. [PubMed] [Google Scholar]
- Linnenbach A. J., Pressler L. B., Seng B. A., Kimmel B. S., Tomaszewski J. E., Malkowicz S. B. Characterization of chromosome 9 deletions in transitional cell carcinoma by microsatellite assay. Hum Mol Genet. 1993 Sep;2(9):1407–1411. doi: 10.1093/hmg/2.9.1407. [DOI] [PubMed] [Google Scholar]
- Mok S. C., Bell D. A., Knapp R. C., Fishbaugh P. M., Welch W. R., Muto M. G., Berkowitz R. S., Tsao S. W. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993 Apr 1;53(7):1489–1492. [PubMed] [Google Scholar]
- Olopade O. I., Bohlander S. K., Pomykala H., Maltepe E., Van Melle E., Le Beau M. M., Diaz M. O. Mapping of the shortest region of overlap of deletions of the short arm of chromosome 9 associated with human neoplasia. Genomics. 1992 Oct;14(2):437–443. doi: 10.1016/s0888-7543(05)80238-1. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Buchhagen D. L., Malik K., Sherman J., Nobori T., Bader S., Nau M. M., Gazdar A. F., Minna J. D., Diaz M. O. Homozygous loss of the interferon genes defines the critical region on 9p that is deleted in lung cancers. Cancer Res. 1993 May 15;53(10 Suppl):2410–2415. [PubMed] [Google Scholar]
- Powell D. E., Puls L., van Nagell J., Jr Current concepts in epithelial ovarian tumors: does benign to malignant transformation occur? Hum Pathol. 1992 Aug;23(8):846–847. doi: 10.1016/0046-8177(92)90393-h. [DOI] [PubMed] [Google Scholar]
- Saito S., Saito H., Koi S., Sagae S., Kudo R., Saito J., Noda K., Nakamura Y. Fine-scale deletion mapping of the distal long arm of chromosome 6 in 70 human ovarian cancers. Cancer Res. 1992 Oct 15;52(20):5815–5817. [PubMed] [Google Scholar]
- Sato T., Saito H., Morita R., Koi S., Lee J. H., Nakamura Y. Allelotype of human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5118–5122. [PubMed] [Google Scholar]
- Teneriello M. G., Ebina M., Linnoila R. I., Henry M., Nash J. D., Park R. C., Birrer M. J. p53 and Ki-ras gene mutations in epithelial ovarian neoplasms. Cancer Res. 1993 Jul 1;53(13):3103–3108. [PubMed] [Google Scholar]
- Vandamme B., Lissens W., Amfo K., De Sutter P., Bourgain C., Vamos E., De Grève J. Deletion of chromosome 11p13-11p15.5 sequences in invasive human ovarian cancer is a subclonal progression factor. Cancer Res. 1992 Dec 1;52(23):6646–6652. [PubMed] [Google Scholar]
- Ward B. G., Wallace K., Shepherd J. H., Balkwill F. R. Intraperitoneal xenografts of human epithelial ovarian cancer in nude mice. Cancer Res. 1987 May 15;47(10):2662–2667. [PubMed] [Google Scholar]
- Weil D., Mattei M. G., Passage E., N'Guyen V. C., Pribula-Conway D., Mann K., Deutzmann R., Timpl R., Chu M. L. Cloning and chromosomal localization of human genes encoding the three chains of type VI collagen. Am J Hum Genet. 1988 Mar;42(3):435–445. [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wilson A. P., Ford C. H., Newman C. E., Howell A. cis-platinum and ovarian carcinoma. In vitro chemosensitivity of cultured tumour cells from patients receiving high dose cis-platinum as first line treatment. Br J Cancer. 1987 Dec;56(6):763–773. doi: 10.1038/bjc.1987.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C. R., Hayward I. P., Lawrie S. S., Buckton K., McIntyre M. A., Adams D. J., Lewis A. D., Scott A. R., Smyth J. F. Cellular heterogeneity and drug resistance in two ovarian adenocarcinoma cell lines derived from a single patient. Int J Cancer. 1987 Jun 15;39(6):695–702. doi: 10.1002/ijc.2910390607. [DOI] [PubMed] [Google Scholar]
- Woods L. K., Morgan R. T., Quinn L. A., Moore G. E., Semple T. U., Stedman K. E. Comparison of four new cell lines from patients with adenocarcinoma of the ovary. Cancer Res. 1979 Nov;39(11):4449–4459. [PubMed] [Google Scholar]
- Yang-Feng T. L., Han H., Chen K. C., Li S. B., Claus E. B., Carcangiu M. L., Chambers S. K., Chambers J. T., Schwartz P. E. Allelic loss in ovarian cancer. Int J Cancer. 1993 Jun 19;54(4):546–551. doi: 10.1002/ijc.2910540405. [DOI] [PubMed] [Google Scholar]
- van Haaften-Day C., Russell P., Rugg C., Wills E. J., Tattersall M. H. Flow cytometric and morphological studies of ovarian carcinoma cell lines and xenografts. Cancer Res. 1983 Aug;43(8):3725–3731. [PubMed] [Google Scholar]