Abstract
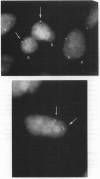
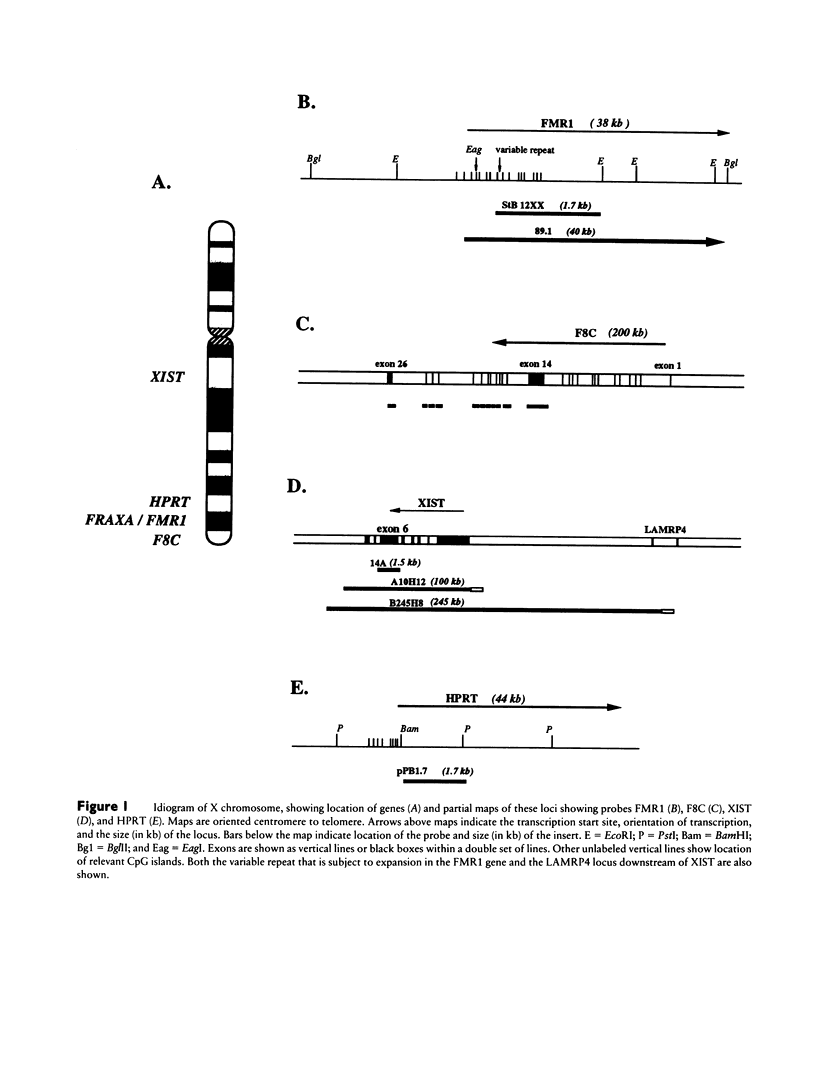
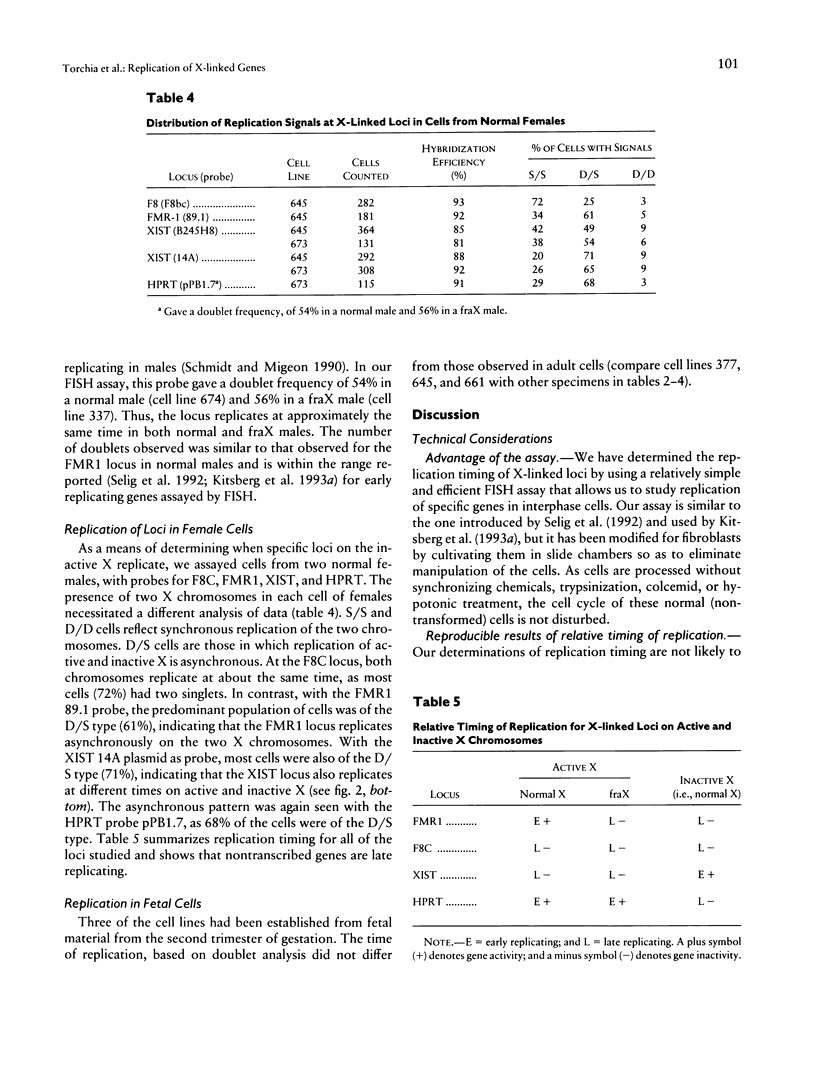
The relationship between the transcriptional state of a locus and the time when it replicates during DNA synthesis is increasingly apparent. Active autosomal genes tend to replicate early, whereas inactive ones are more permissive and frequently replicate later. Although the inactive X chromosome replicates later than its active homologue, little is known about the replication of X-linked genes. We have used FISH to examine the replication of loci on the active X chromosome that are not transcribed, either because the tissue analyzed was not the expressing tissue (F8C), because the locus is silent on all active X chromosomes (XIST), or because it has been mutated by expansion and methylation of a CpG island (FMR1). In this assay, an unreplicated locus is characterized by a single hybridization signal, and a replicated locus is characterized by a doublet hybridization signal. The percentage of doublets is used as a measure of relative time of replication in S phase. The validity of this approach has been established elsewhere, since results compare favorably with those obtained using traditional methods for studying DNA replication. Our results show that the FMR1 gene replicates relatively later in fragile X (fraX) males with the full mutation than in normal males, irrespective of the probe used. The F8C locus is late replicating in both normal and fraX males and replicates at nearly the same time on active and inactive X in females. The XIST locus replicates late in all the males studied and asynchronously in female cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballabio A., Willard H. F. Mammalian X-chromosome inactivation and the XIST gene. Curr Opin Genet Dev. 1992 Jun;2(3):439–447. doi: 10.1016/s0959-437x(05)80155-8. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Ballabio A., Rupert J. L., Lafreniere R. G., Grompe M., Tonlorenzi R., Willard H. F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991 Jan 3;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Richards S., Gibbs R. A., Nelson D. L. Fine structure of the human FMR1 gene. Hum Mol Genet. 1993 Aug;2(8):1147–1153. doi: 10.1093/hmg/2.8.1147. [DOI] [PubMed] [Google Scholar]
- Furst A., Brown E. H., Braunstein J. D., Schildkraut C. L. alpha-Globulin sequences are located in a region of early-replicating DNA in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1023–1027. doi: 10.1073/pnas.78.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. M. Temporal order of replication of Xenopus laevis 5S ribosomal RNA genes in somatic cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2924–2928. doi: 10.1073/pnas.83.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Grumbach M. M., Morishima A., Taylor J. H. HUMAN SEX CHROMOSOME ABNORMALITIES IN RELATION TO DNA REPLICATION AND HETEROCHROMATINIZATION. Proc Natl Acad Sci U S A. 1963 May;49(5):581–589. doi: 10.1073/pnas.49.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R. S., Canfield T. K., Lamb M. M., Gartler S. M., Laird C. D. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell. 1993 Jul 2;73(7):1403–1409. doi: 10.1016/0092-8674(93)90365-w. [DOI] [PubMed] [Google Scholar]
- Hendrich B. D., Brown C. J., Willard H. F. Evolutionary conservation of possible functional domains of the human and murine XIST genes. Hum Mol Genet. 1993 Jun;2(6):663–672. doi: 10.1093/hmg/2.6.663. [DOI] [PubMed] [Google Scholar]
- Holmquist G. P. Role of replication time in the control of tissue-specific gene expression. Am J Hum Genet. 1987 Feb;40(2):151–173. [PMC free article] [PubMed] [Google Scholar]
- Holmquist G., Gray M., Porter T., Jordan J. Characterization of Giemsa dark- and light-band DNA. Cell. 1982 Nov;31(1):121–129. doi: 10.1016/0092-8674(82)90411-1. [DOI] [PubMed] [Google Scholar]
- Jolly D. J., Esty A. C., Bernard H. U., Friedmann T. Isolation of a genomic clone partially encoding human hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5038–5041. doi: 10.1073/pnas.79.16.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsberg D., Selig S., Brandeis M., Simon I., Keshet I., Driscoll D. J., Nicholls R. D., Cedar H. Allele-specific replication timing of imprinted gene regions. Nature. 1993 Jul 29;364(6436):459–463. doi: 10.1038/364459a0. [DOI] [PubMed] [Google Scholar]
- Kitsberg D., Selig S., Keshet I., Cedar H. Replication structure of the human beta-globin gene domain. Nature. 1993 Dec 9;366(6455):588–590. doi: 10.1038/366588a0. [DOI] [PubMed] [Google Scholar]
- Knoll J. H., Cheng S. D., Lalande M. Allele specificity of DNA replication timing in the Angelman/Prader-Willi syndrome imprinted chromosomal region. Nat Genet. 1994 Jan;6(1):41–46. doi: 10.1038/ng0194-41. [DOI] [PubMed] [Google Scholar]
- Lafrenière R. G., Brown C. J., Rider S., Chelly J., Taillon-Miller P., Chinault A. C., Monaco A. P., Willard H. F. 2.6 Mb YAC contig of the human X inactivation center region in Xq13: physical linkage of the RPS4X, PHKA1, XIST and DXS128E genes. Hum Mol Genet. 1993 Aug;2(8):1105–1115. doi: 10.1093/hmg/2.8.1105. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Green E. D., Cremer T. Fluorescence in situ hybridization of YAC clones after Alu-PCR amplification. Genomics. 1992 Jul;13(3):826–828. doi: 10.1016/0888-7543(92)90160-t. [DOI] [PubMed] [Google Scholar]
- Luo S., Robinson J. C., Reiss A. L., Migeon B. R. DNA methylation of the fragile X locus in somatic and germ cells during fetal development: relevance to the fragile X syndrome and X inactivation. Somat Cell Mol Genet. 1993 Jul;19(4):393–404. doi: 10.1007/BF01232750. [DOI] [PubMed] [Google Scholar]
- Miller D. R., Dewey W. C., Miller H. H. X-ray-induced delay in the Chinese hamster cell-cycle: dependence on phase irradiated under different culturing conditions, BUdR incorporation, and hypertonic treatment. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Jun;23(6):591–602. doi: 10.1080/09553007314550691. [DOI] [PubMed] [Google Scholar]
- Richler C., Soreq H., Wahrman J. X inactivation in mammalian testis is correlated with inactive X-specific transcription. Nat Genet. 1992 Nov;2(3):192–195. doi: 10.1038/ng1192-192. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Oberlé I., Mandel J. L. On some technical aspects of direct DNA diagnosis of the fragile X syndrome. 1992 Apr 15-May 1Am J Med Genet. 43(1-2):197–207. doi: 10.1002/ajmg.1320430133. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Migeon B. R. Asynchronous replication of homologous loci on human active and inactive X chromosomes. Proc Natl Acad Sci U S A. 1990 May;87(10):3685–3689. doi: 10.1073/pnas.87.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig S., Okumura K., Ward D. C., Cedar H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J. 1992 Mar;11(3):1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR J. H. Asynchronous duplication of chromosomes in cultured cells of Chinese hamster. J Biophys Biochem Cytol. 1960 Jun;7:455–464. doi: 10.1083/jcb.7.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Trask B., Pinkel D., van den Engh G. The proximity of DNA sequences in interphase cell nuclei is correlated to genomic distance and permits ordering of cosmids spanning 250 kilobase pairs. Genomics. 1989 Nov;5(4):710–717. doi: 10.1016/0888-7543(89)90112-2. [DOI] [PubMed] [Google Scholar]
- Webb T. Delayed replication of Xq27 in individuals with the fragile X syndrome. Am J Med Genet. 1992 Aug 1;43(6):1057–1062. doi: 10.1002/ajmg.1320430633. [DOI] [PubMed] [Google Scholar]
- Wion K. L., Kelly D., Summerfield J. A., Tuddenham E. G., Lawn R. M. Distribution of factor VIII mRNA and antigen in human liver and other tissues. Nature. 1985 Oct 24;317(6039):726–729. doi: 10.1038/317726a0. [DOI] [PubMed] [Google Scholar]