Abstract
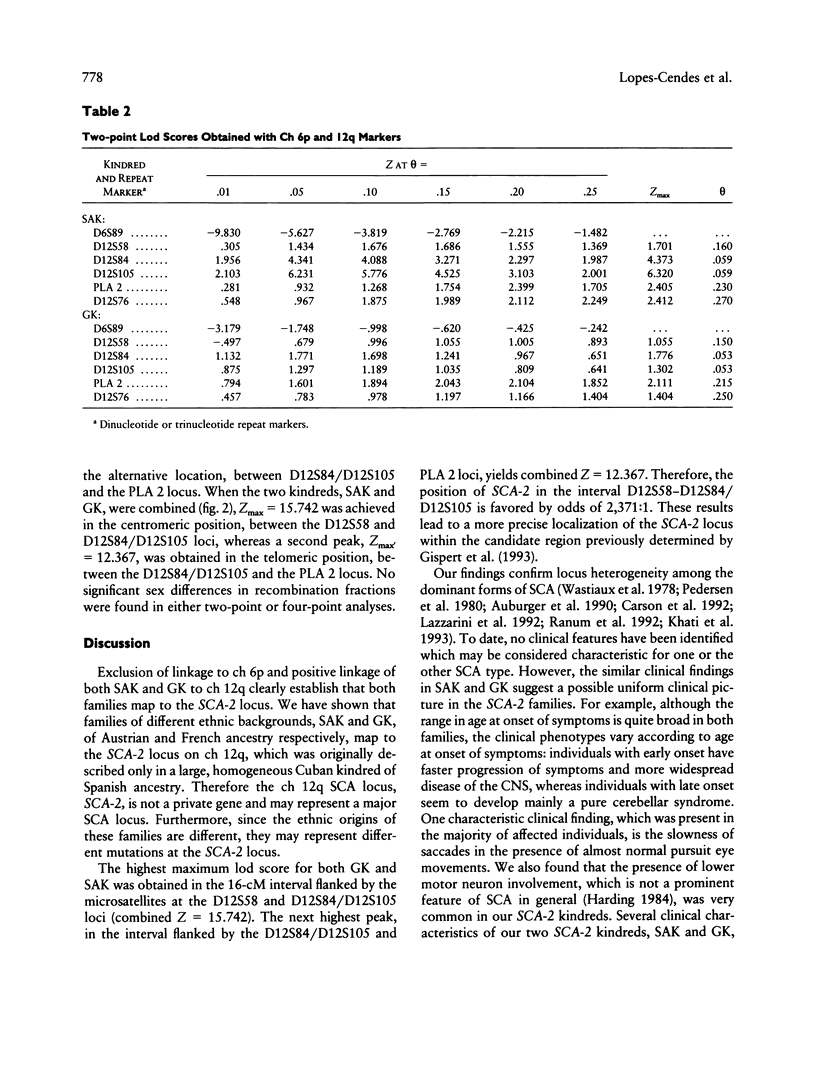
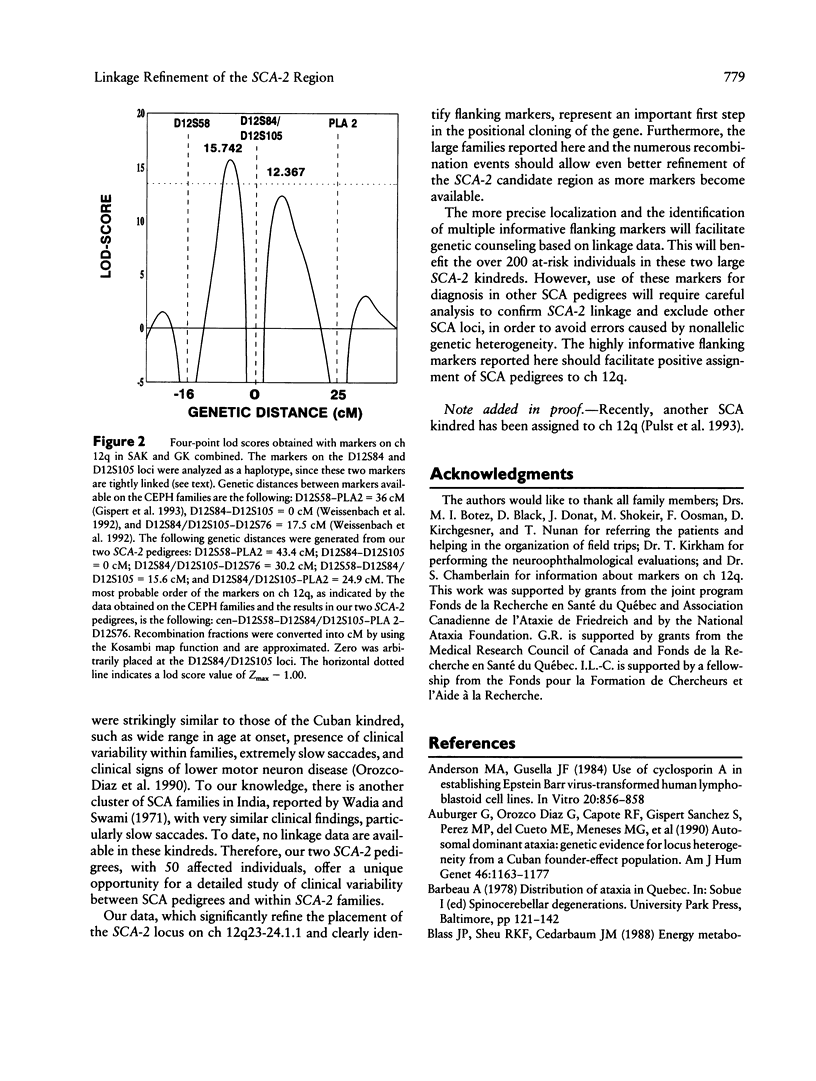
The autosomal dominant spinocerebellar ataxias (SCAs) are a clinically heterogeneous group of neurodegenerative diseases. To date, two SCA loci have been identified-one locus (SCA-1) on the short arm of chromosome 6 and the second locus (SCA-2) on the long arm of chromosome 12. We have studied two large kindreds from different ethnic backgrounds, segregating an autosomal dominant form of SCA. A total of 207 living individuals, including 50 affected, were examined, and blood was collected. We performed linkage analysis using anonymous DNA markers which flank the two previously described loci. Our results demonstrate that the two kindreds, one Austrian-Canadian and one French-Canadian, are linked to SCA-2 (chromosome 12q). Multipoint linkage analysis places the SCA-2 locus within a region of approximately 16 cM between the microsatellites D12S58 and D12S84/D12S105 (odds ratio 2,371:1 in favor of this position). We show that the SCA-2 locus is not a private gene and represents an alternative SCA locus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Auburger G., Diaz G. O., Capote R. F., Sanchez S. G., Perez M. P., del Cueto M. E., Meneses M. G., Farrall M., Williamson R., Chamberlain S. Autosomal dominant ataxia: genetic evidence for locus heterogeneity from a Cuban founder-effect population. Am J Hum Genet. 1990 Jun;46(6):1163–1177. [PMC free article] [PubMed] [Google Scholar]
- Blass J. P., Sheu R. K., Cedarbaum J. M. Energy metabolism in disorders of the nervous system. Rev Neurol (Paris) 1988;144(10):543–563. [PubMed] [Google Scholar]
- Carson W. J., Radvany J., Farrer L. A., Vincent D., Rosenberg R. N., MacLeod P. M., Rouleau G. A. The Machado-Joseph disease locus is different from the spinocerebellar ataxia locus (SCA1). Genomics. 1992 Jul;13(3):852–855. doi: 10.1016/0888-7543(92)90168-r. [DOI] [PubMed] [Google Scholar]
- Duvoisin R. C., Chokroverty S., Lepore F., Nicklas W. Glutamate dehydrogenase deficiency in patients with olivopontocerebellar atrophy. Neurology. 1983 Oct;33(10):1322–1326. doi: 10.1212/wnl.33.10.1332. [DOI] [PubMed] [Google Scholar]
- Frontali M., Iodice C., Lulli P., Spadaro M., Cappellacci S., Giunti P., Malaspina P., Morellini M., Morocutti C., Novelletto A. Spinocerebellar ataxia (SCA1) in two large Italian kindreds: evidence in favour of a locus position distal to GLO1 and the HLA cluster. Ann Hum Genet. 1991 Jan;55(Pt 1):7–15. doi: 10.1111/j.1469-1809.1991.tb00393.x. [DOI] [PubMed] [Google Scholar]
- GERSTENBRAND F., WEINGARTEN K. [Contribution to the problem of familial atrophy of the cerebellum]. Wien Klin Wochenschr. 1962 Oct 12;74:702–705. [PubMed] [Google Scholar]
- Gispert S., Twells R., Orozco G., Brice A., Weber J., Heredero L., Scheufler K., Riley B., Allotey R., Nothers C. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet. 1993 Jul;4(3):295–299. doi: 10.1038/ng0793-295. [DOI] [PubMed] [Google Scholar]
- Gudmundsson K. R. Prevalence and occurrence of some rare neurological diseases in Iceland. Acta Neurol Scand. 1969;45(1):114–118. doi: 10.1111/j.1600-0404.1969.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Haines J. L., Schut L. J., Weitkamp L. R., Thayer M., Anderson V. E. Spinocerebellar ataxia in a large kindred: age at onset, reproduction, and genetic linkage studies. Neurology. 1984 Dec;34(12):1542–1548. doi: 10.1212/wnl.34.12.1542. [DOI] [PubMed] [Google Scholar]
- Jackson J. F., Currier R. D., Terasaki P. I., Morton N. E. Spinocerebellar ataxia and HLA linkage: risk prediction by HLA typing. N Engl J Med. 1977 May 19;296(20):1138–1141. doi: 10.1056/NEJM197705192962003. [DOI] [PubMed] [Google Scholar]
- Jackson J. F., Whittington J. E., Currier R. D., Terasaki P. I., Morton N. E., Keats B. J. Genetic linkage and spinocerebellar ataxia. Adv Neurol. 1978;21:315–318. [PubMed] [Google Scholar]
- Keats B. J., Pollack M. S., McCall A., Wilensky M. A., Ward L. J., Lu M., Zoghbi H. Y. Tight linkage of the gene for spinocerebellar ataxia to D6S89 on the short arm of chromosome 6 in a kindred for which close linkage to both HLA and F13A1 is excluded. Am J Hum Genet. 1991 Nov;49(5):972–977. [PMC free article] [PubMed] [Google Scholar]
- Khati C., Stevanin G., Durr A., Chneiweiss H., Belal S., Seck A., Cann H., Brice A., Agid Y. Genetic heterogeneity of autosomal dominant cerebellar ataxia type 1: clinical and genetic analysis of 10 French families. Neurology. 1993 Jun;43(6):1131–1137. doi: 10.1212/wnl.43.6.1131. [DOI] [PubMed] [Google Scholar]
- Kish S. J., Currier R. D., Schut L., Perry T. L., Morito C. L. Brain choline acetyltransferase reduction in dominantly inherited olivopontocerebellar atrophy. Ann Neurol. 1987 Aug;22(2):272–275. doi: 10.1002/ana.410220214. [DOI] [PubMed] [Google Scholar]
- Koeppen A. H., Goedde H. W., Hirth L., Benkmann H. G., Hiller C. Genetic linkage in hereditary ataxia. Lancet. 1980 Jan 12;1(8159):92–93. doi: 10.1016/s0140-6736(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Kumar D., Blank C. E., Gelsthorpe K. Hereditary cerebellar ataxia and genetic linkage with HLA. Hum Genet. 1986 Apr;72(4):327–332. doi: 10.1007/BF00290959. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini A., Zimmerman T. R., Jr, Johnson W. G., Duvoisin R. C. A 17th-century founder gives rise to a large north American pedigree of autosomal dominant spinocerebellar ataxia not linked to the SCA1 locus on chromosome 6. Neurology. 1992 Nov;42(11):2118–2124. doi: 10.1212/wnl.42.11.2118. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. Dinucleotide repeat polymorphism at the D6S89 locus. Nucleic Acids Res. 1990 Jul 25;18(14):4301–4301. doi: 10.1093/nar/18.14.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyam B. V., Giacobini E., Ferraro T. N., Hare T. A. Cerebrospinal fluid as a reflector of central cholinergic and amino acid neurotransmitter activity in cerebellar ataxia. Arch Neurol. 1990 Nov;47(11):1194–1199. doi: 10.1001/archneur.1990.00530110048016. [DOI] [PubMed] [Google Scholar]
- Möller E., Hindfelt B., Olsson J. E. HLA--determination in families with hereditary ataxia. Tissue Antigens. 1978 Nov;12(5):357–366. doi: 10.1111/j.1399-0039.1978.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Nancarrow D. J., Walker G. J., Weber J. L., Walters M. K., Palmer J. M., Hayward N. K. Linkage mapping of melanoma (MLM) using 172 microsatellite markers. Genomics. 1992 Dec;14(4):939–947. doi: 10.1016/s0888-7543(05)80115-6. [DOI] [PubMed] [Google Scholar]
- Nino H. E., Noreen H. J., Dubey D. P., Resch J. A., Namboodiri K., Elston R. C., Yunis E. J. A family with hereditary ataxia: HLA typing. Neurology. 1980 Jan;30(1):12–20. doi: 10.1212/wnl.30.1.12. [DOI] [PubMed] [Google Scholar]
- Orozco Diaz G., Nodarse Fleites A., Cordovés Sagaz R., Auburger G. Autosomal dominant cerebellar ataxia: clinical analysis of 263 patients from a homogeneous population in Holguín, Cuba. Neurology. 1990 Sep;40(9):1369–1375. doi: 10.1212/wnl.40.9.1369. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Ott J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am J Hum Genet. 1974 Sep;26(5):588–597. [PMC free article] [PubMed] [Google Scholar]
- Pedersen L., Platz P., Ryder L. P., Lamm L. U., Dissing J. A linkage study of hereditary ataxias and related disorders. Evidence of heterogeneity of dominant cerebellar ataxia. Hum Genet. 1980;54(3):371–383. doi: 10.1007/BF00291585. [DOI] [PubMed] [Google Scholar]
- Pedraza O. L., Botez M. I. Thiamine status in inherited degenerative ataxias. J Neurol Neurosurg Psychiatry. 1992 Feb;55(2):136–137. doi: 10.1136/jnnp.55.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Rath D. S., Xiao H., Merril C. R. Trinucleotide repeat polymorphism at the human pancreatic phospholipase A-2 gene (PLA2). Nucleic Acids Res. 1990 Dec 25;18(24):7468–7468. [PMC free article] [PubMed] [Google Scholar]
- Pulst S. M., Nechiporuk A., Starkman S. Anticipation in spinocerebellar ataxia type 2. Nat Genet. 1993 Sep;5(1):8–10. doi: 10.1038/ng0993-8c. [DOI] [PubMed] [Google Scholar]
- Ranum L. P., Duvick L. A., Rich S. S., Schut L. J., Litt M., Orr H. T. Localization of the autosomal dominant HLA-linked spinocerebellar ataxia (SCA1) locus, in two kindreds, within an 8-cM subregion of chromosome 6p. Am J Hum Genet. 1991 Jul;49(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- Ranum L. P., Rich S. S., Nance M. A., Duvick L. A., Aita J. F., Orr H. T., Anton-Johnson S., Schut L. J. Autosomal dominant spinocerebellar ataxia: locus heterogeneity in a Nebraska kindred. Neurology. 1992 Feb;42(2):344–347. doi: 10.1212/wnl.42.2.344. [DOI] [PubMed] [Google Scholar]
- Rich S. S., Wilkie P., Schut L., Vance G., Orr H. T. Spinocerebellar ataxia: localization of an autosomal dominant locus between two markers on human chromosome 6. Am J Hum Genet. 1987 Oct;41(4):524–531. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. N. Autosomal dominant cerebellar phenotypes: the genotype will settle the issue. Neurology. 1990 Sep;40(9):1329–1331. doi: 10.1212/wnl.40.9.1329. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Nishizawa M., Tanaka H., Kawashima S., Sakamoto H., Karube Y., Shimazaki H., Soutome M., Endo K., Ohta S. The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet. 1993 Jul;4(3):300–304. doi: 10.1038/ng0793-300. [DOI] [PubMed] [Google Scholar]
- Wadia N. H., Swami R. K. A new form of heredo-familial spinocerebellar degeneration with slow eye movements (nine families). Brain. 1971;94(2):359–374. doi: 10.1093/brain/94.2.359. [DOI] [PubMed] [Google Scholar]
- Wastiaux J. P., Lamoureux G., Bouchard J. P., Durivage A., Barbeau C., Barbeau A. HLA and complement typing in olivo-ponto-cerebellar atrophy. Can J Neurol Sci. 1978 Feb;5(1):75–81. [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Werdelin L., Keiding N. Hereditary ataxias: epidemiological aspects. Neuroepidemiology. 1990;9(6):321–331. doi: 10.1159/000110795. [DOI] [PubMed] [Google Scholar]
- Wilkie P. J., Schut L. J., Rich S. S. Spinocerebellar ataxia: multipoint linkage analysis of genes associated with the disease locus. Hum Genet. 1991 Aug;87(4):405–408. doi: 10.1007/BF00197157. [DOI] [PubMed] [Google Scholar]
- Yakura H., Wakisaka A., Fujimoto S., Itakura K. Letter: Hereditary ataxia and HL-A. N Engl J Med. 1974 Jul 18;291(3):154–155. doi: 10.1056/NEJM197407182910314. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Jodice C., Sandkuijl L. A., Kwiatkowski T. J., Jr, McCall A. E., Huntoon S. A., Lulli P., Spadaro M., Litt M., Cann H. M. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps telomeric to the HLA complex and is closely linked to the D6S89 locus in three large kindreds. Am J Hum Genet. 1991 Jul;49(1):23–30. [PMC free article] [PubMed] [Google Scholar]


