Abstract
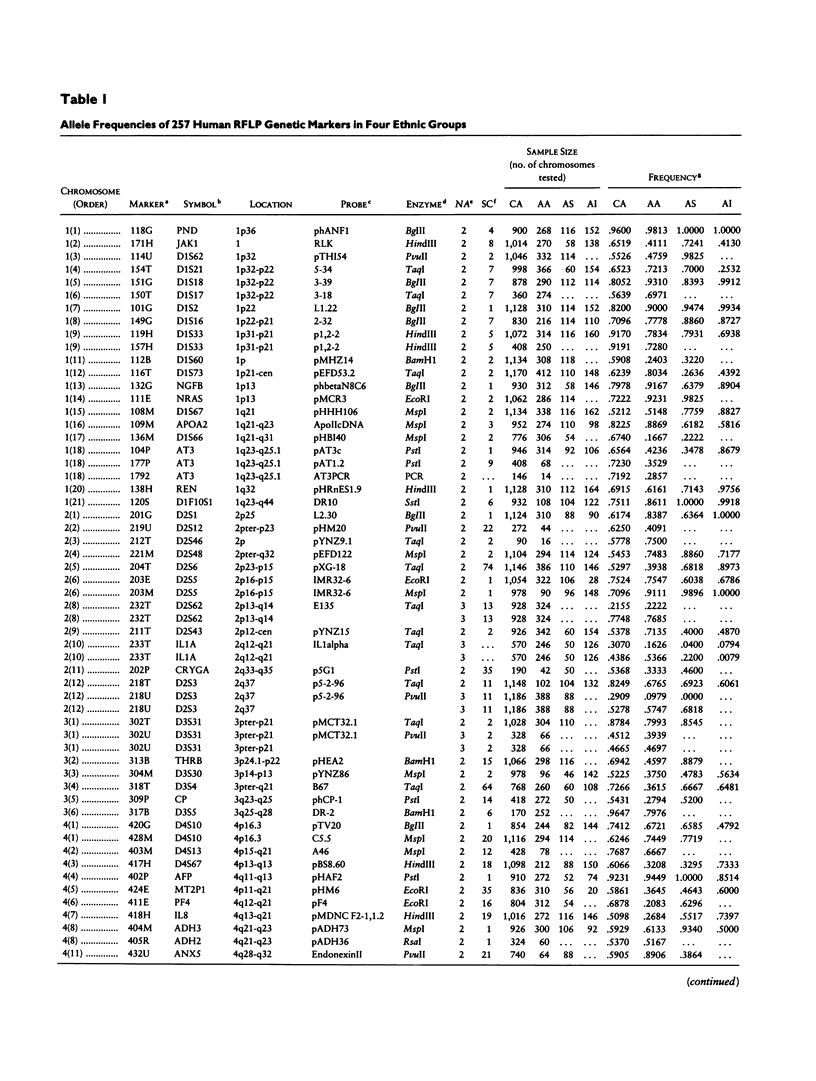
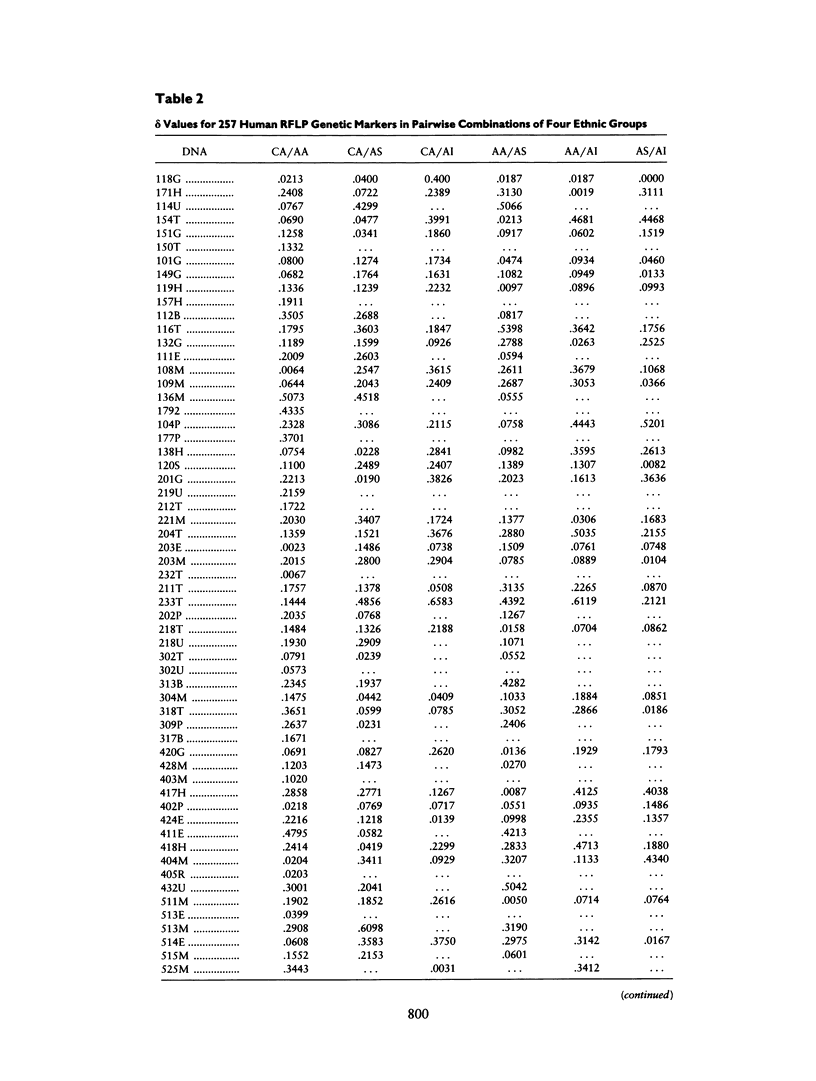
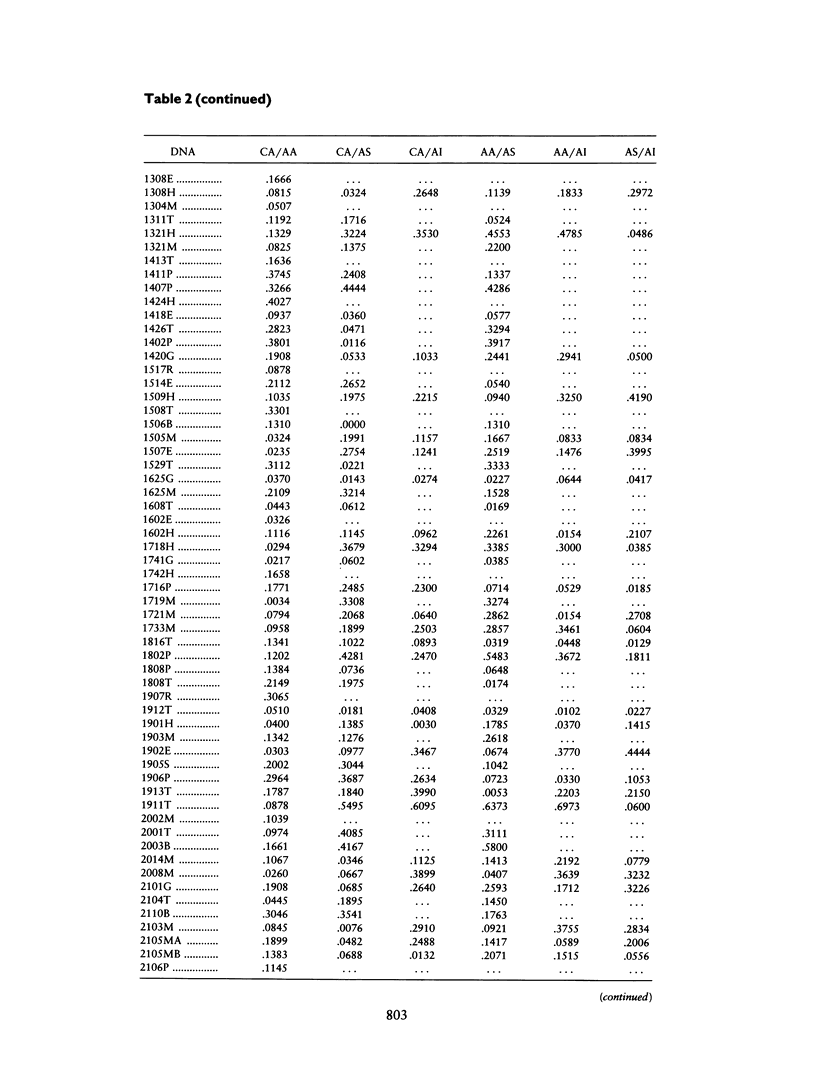
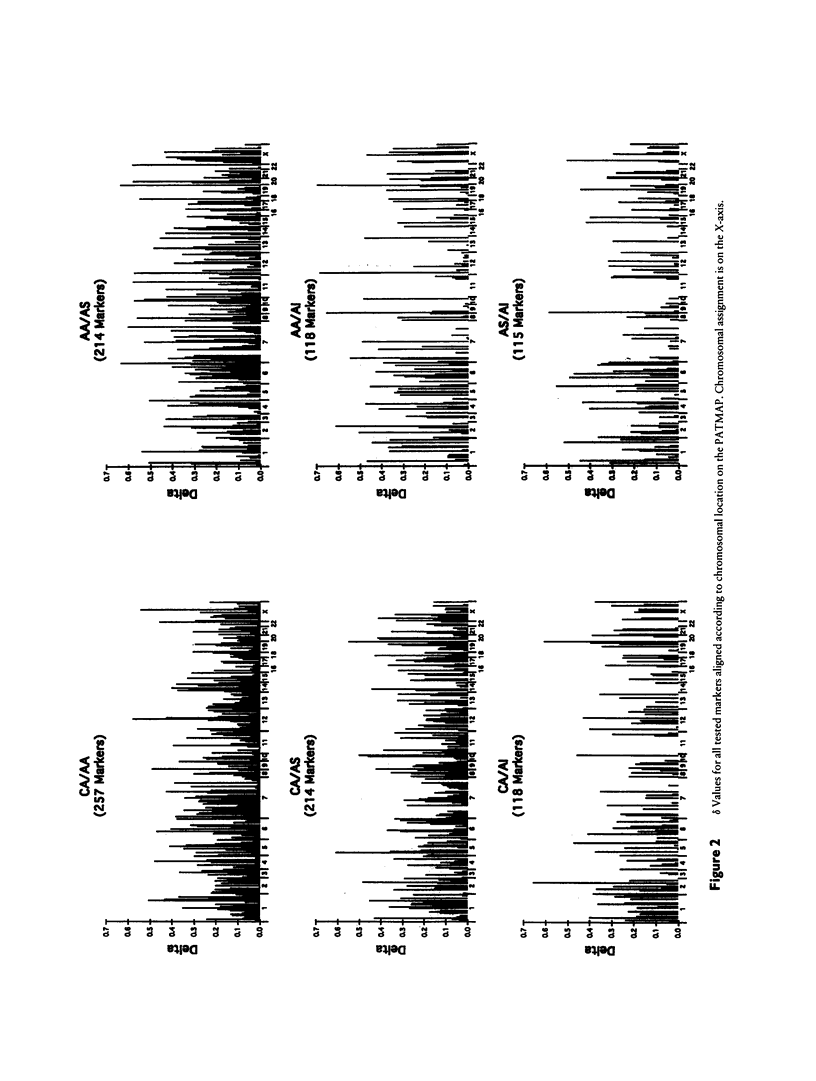
A panel of 257 RFLP loci was selected on the basis of high heterozygosity in Caucasian DNA surveys and equivalent spacing throughout the human genome. Probes from each locus were used in a Southern blot survey of allele frequency distribution for four human ethnic groups: Caucasian, African American, Asian (Chinese), and American Indian (Cheyenne). Nearly all RFLP loci were polymorphic in each group, albeit with a broad range of differing allele frequencies (δ). The distribution of frequency differences (δ values) was used for three purposes: (1) to provide estimates for genetic distance (differentiation) among these ethnic groups, (2) to revisit with a large data set the proportion of human genetic variation attributable to differentiation within ethnic groups, and (3) to identify loci with high δ values between recently admixed populations of use in mapping by admixture linkage disequilibrium (MALD). Although most markers display significant allele frequency differences between ethnic groups, the overall genetic distances between ethnic groups were small (.066–.098), and <10% of the measured overall molecular genetic diversity in these human samples can be attributed to “racial” differentiation. The median δ values for pairwise comparisons between groups fell between .15 and .20, permitting identification of highly informative RFLP loci for MALD disease association studies.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Bowcock A. M., Bucci C., Hebert J. M., Kidd J. R., Kidd K. K., Friedlaender J. S., Cavalli-Sforza L. L. Study of 47 DNA markers in five populations from four continents. Gene Geogr. 1987 Apr;1(1):47–64. [PubMed] [Google Scholar]
- Bowcock A. M., Kidd J. R., Mountain J. L., Hebert J. M., Carotenuto L., Kidd K. K., Cavalli-Sforza L. L. Drift, admixture, and selection in human evolution: a study with DNA polymorphisms. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):839–843. doi: 10.1073/pnas.88.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe D., Stephens J. C., O'Brien S. J. Linkage disequilibrium in admixed populations: applications in gene mapping. J Hered. 1994 Jan-Feb;85(1):59–63. [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L. L., Menozzi P., Piazza A. Demic expansions and human evolution. Science. 1993 Jan 29;259(5095):639–646. doi: 10.1126/science.8430313. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Weiss K. M. Admixture as a tool for finding linked genes and detecting that difference from allelic association between loci. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9119–9123. doi: 10.1073/pnas.85.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F., Galas D. A new five-year plan for the U.S. Human Genome Project. Science. 1993 Oct 1;262(5130):43–46. doi: 10.1126/science.8211127. [DOI] [PubMed] [Google Scholar]
- Cuticchia A. J., Chipperfield M. A., Porter C. J., Kearns W., Pearson P. L. Managing all those bytes: the Human Genome Project. Science. 1993 Oct 1;262(5130):47–48. doi: 10.1126/science.8211128. [DOI] [PubMed] [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992 Feb;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Gibbons A. Geneticists trace the DNA trail of the first Americans. Science. 1993 Jan 15;259(5093):312–313. doi: 10.1126/science.8420001. [DOI] [PubMed] [Google Scholar]
- Ginzburg H. M., Fleming P. L., Miller K. D. Selected public health observations derived from Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1988;1(1):2–7. [PubMed] [Google Scholar]
- Goedert J. J., Biggar R. J., Weiss S. H., Eyster M. E., Melbye M., Wilson S., Ginzburg H. M., Grossman R. J., DiGioia R. A., Sanchez W. C. Three-year incidence of AIDS in five cohorts of HTLV-III-infected risk group members. Science. 1986 Feb 28;231(4741):992–995. doi: 10.1126/science.3003917. [DOI] [PubMed] [Google Scholar]
- Goedert J. J., Kessler C. M., Aledort L. M., Biggar R. J., Andes W. A., White G. C., 2nd, Drummond J. E., Vaidya K., Mann D. L., Eyster M. E. A prospective study of human immunodeficiency virus type 1 infection and the development of AIDS in subjects with hemophilia. N Engl J Med. 1989 Oct 26;321(17):1141–1148. doi: 10.1056/NEJM198910263211701. [DOI] [PubMed] [Google Scholar]
- Hadler S. C., Francis D. P., Maynard J. E., Thompson S. E., Judson F. N., Echenberg D. F., Ostrow D. G., O'Malley P. M., Penley K. A., Altman N. L. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986 Jul 24;315(4):209–214. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- Hudson R. R., Kaplan N. L. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics. 1985 Sep;111(1):147–164. doi: 10.1093/genetics/111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R. R. Properties of a neutral allele model with intragenic recombination. Theor Popul Biol. 1983 Apr;23(2):183–201. doi: 10.1016/0040-5809(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Klein J., Takahata N., Ayala F. J. MHC polymorphism and human origins. Sci Am. 1993 Dec;269(6):78–83. doi: 10.1038/scientificamerican1293-78. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Roberts L. Genome Diversity Project. Anthropologists climb (gingerly) on board. Science. 1992 Nov 20;258(5086):1300–1301. doi: 10.1126/science.1455222. [DOI] [PubMed] [Google Scholar]
- Schurr T. G., Ballinger S. W., Gan Y. Y., Hodge J. A., Merriwether D. A., Lawrence D. N., Knowler W. C., Weiss K. M., Wallace D. C. Amerindian mitochondrial DNAs have rare Asian mutations at high frequencies, suggesting they derived from four primary maternal lineages. Am J Hum Genet. 1990 Mar;46(3):613–623. [PMC free article] [PubMed] [Google Scholar]
- Stephens J. C., Briscoe D., O'Brien S. J. Mapping by admixture linkage disequilibrium in human populations: limits and guidelines. Am J Hum Genet. 1994 Oct;55(4):809–824. [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Evolutionary relationship of DNA sequences in finite populations. Genetics. 1983 Oct;105(2):437–460. doi: 10.1093/genetics/105.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigilant L., Stoneking M., Harpending H., Hawkes K., Wilson A. C. African populations and the evolution of human mitochondrial DNA. Science. 1991 Sep 27;253(5027):1503–1507. doi: 10.1126/science.1840702. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Garrison K., Knowler W. C. Dramatic founder effects in Amerindian mitochondrial DNAs. Am J Phys Anthropol. 1985 Oct;68(2):149–155. doi: 10.1002/ajpa.1330680202. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., Wong C. Mutation of human short tandem repeats. Hum Mol Genet. 1993 Aug;2(8):1123–1128. doi: 10.1093/hmg/2.8.1123. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]


