Abstract
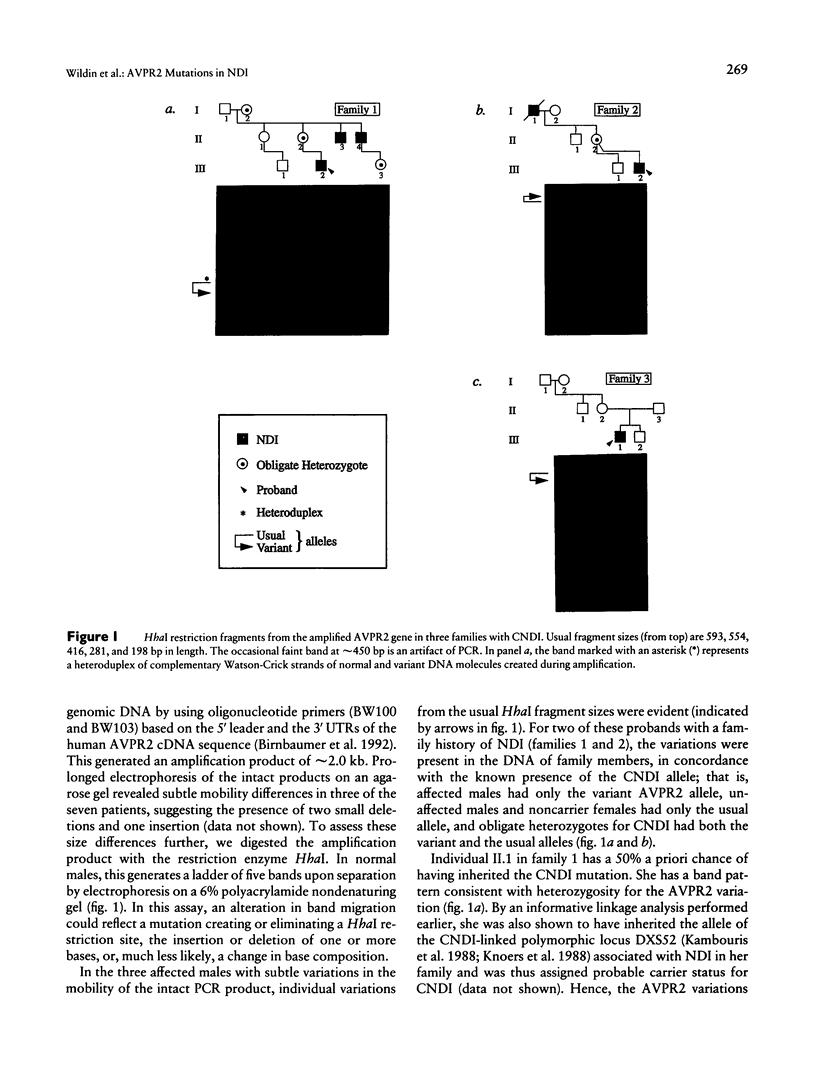
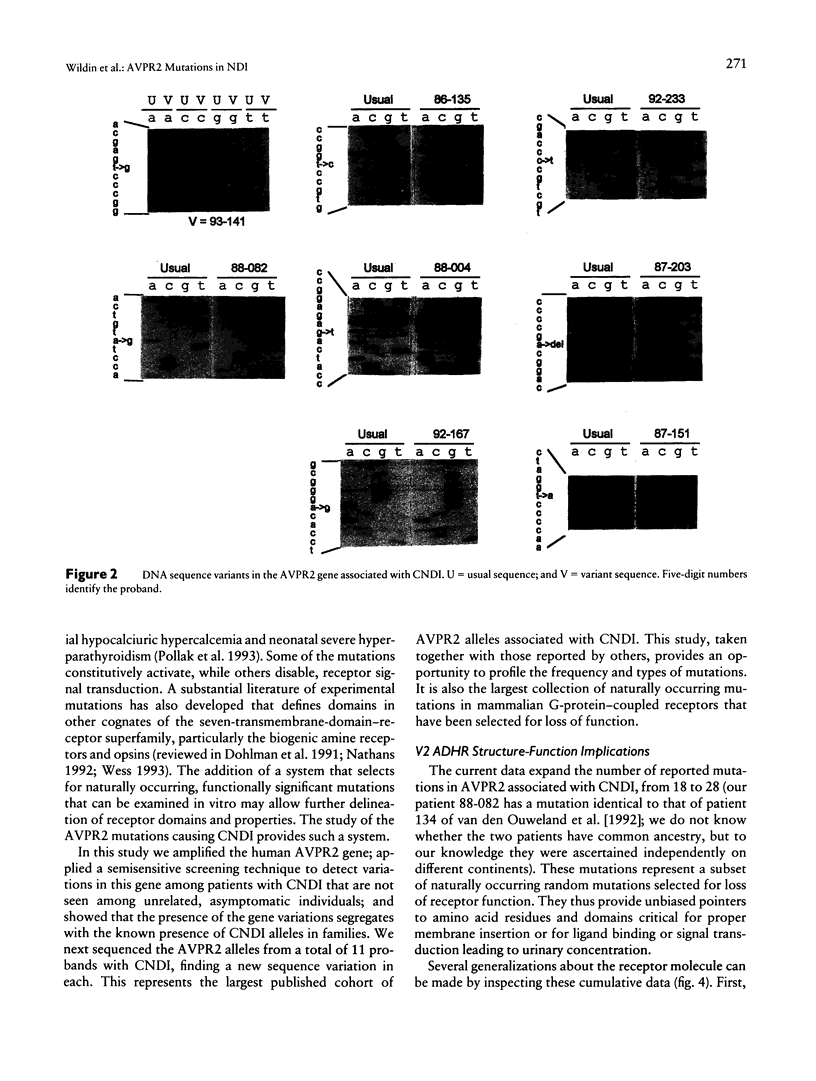
Mutations in the AVPR2 gene encoding the receptor for arginine vasopressin in the kidney (V2 ADHR) have been reported in patients with congenital nephrogenic diabetes insipidus, a predominantly X-linked disorder of water homeostasis. We have used restriction-enzyme analysis and direct DNA sequencing of genomic PCR product to evaluate the AVPR2 gene in 11 unrelated affected males. Each patient has a different DNA sequence variation, and only one matches a previously reported mutation. Cosegregation of the variations with nephrogenic diabetes insipidus was demonstrated for two families, and a de novo mutation was documented in two additional cases. Carrier detection was accomplished in one family. All the variations predict frameshifts, truncations, or nonconservative amino acid substitutions in evolutionarily conserved positions in the V2 ADHR and related receptors. Of interest, a 28-bp deletion is found in one patient, while another, unrelated patient has a tandem duplication of the same 28-bp segment, suggesting that both resulted from the same unusual unequal crossing-over mechanism facilitated by 9-mer direct sequence repeats. Since the V2 ADHR is a member of the seven-transmembrane-domain, G-protein-coupled receptor superfamily, the loss-of-function mutations from this study and others provide important clues to the structure-function relationship of this and related receptors.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bichet D. G., Arthus M. F., Lonergan M., Hendy G. N., Paradis A. J., Fujiwara T. M., Morgan K., Gregory M. C., Rosenthal W., Didwania A. X-linked nephrogenic diabetes insipidus mutations in North America and the Hopewell hypothesis. J Clin Invest. 1993 Sep;92(3):1262–1268. doi: 10.1172/JCI116698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer M., Seibold A., Gilbert S., Ishido M., Barberis C., Antaramian A., Brabet P., Rosenthal W. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992 May 28;357(6376):333–335. doi: 10.1038/357333a0. [DOI] [PubMed] [Google Scholar]
- Bradley T. B., Jr, Wohl R. C., Rieder R. F. Hemoglobin Gun Hill: deletion of five amino acid residues and impaired heme-globin binding. Science. 1967 Sep 29;157(3796):1581–1583. doi: 10.1126/science.157.3796.1581. [DOI] [PubMed] [Google Scholar]
- Braun E. J., Stallone J. N. The occurrence of nephrogenic diabetes insipidus in domestic fowl. Am J Physiol. 1989 Apr;256(4 Pt 2):F639–F645. doi: 10.1152/ajprenal.1989.256.4.F639. [DOI] [PubMed] [Google Scholar]
- Carré-Eusèbe D., Imbeaud S., Harbison M., New M. I., Josso N., Picard J. Y. Variants of the anti-Müllerian hormone gene in a compound heterozygote with the persistent Müllerian duct syndrome and his family. Hum Genet. 1992 Dec;90(4):389–394. doi: 10.1007/BF00220465. [DOI] [PubMed] [Google Scholar]
- Clapham D. E. Mutations in G protein-linked receptors: novel insights on disease. Cell. 1993 Dec 31;75(7):1237–1239. doi: 10.1016/0092-8674(93)90609-t. [DOI] [PubMed] [Google Scholar]
- Clark A. J., McLoughlin L., Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993 Feb 20;341(8843):461–462. doi: 10.1016/0140-6736(93)90208-x. [DOI] [PubMed] [Google Scholar]
- Deeb S. S., Lindsey D. T., Hibiya Y., Sanocki E., Winderickx J., Teller D. Y., Motulsky A. G. Genotype-phenotype relationships in human red/green color-vision defects: molecular and psychophysical studies. Am J Hum Genet. 1992 Oct;51(4):687–700. [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fukuhara Y., Sakuraba H., Oshima A., Shimmoto M., Nagao Y., Nadaoka Y., Suzuki T., Suzuki Y. Partial deletion of human alpha-galactosidase A gene in Fabry disease: direct repeat sequences as a possible cause of slipped mispairing. Biochem Biophys Res Commun. 1990 Jul 16;170(1):296–300. doi: 10.1016/0006-291x(90)91273-u. [DOI] [PubMed] [Google Scholar]
- Holtzman E. J., Kolakowski L. F., Jr, O'Brien D., Crawford J. D., Ausiello D. A. A Null mutation in the vasopressin V2 receptor gene (AVPR2) associated with nephrogenic diabetes insipidus in the Hopewell kindred. Hum Mol Genet. 1993 Aug;2(8):1201–1204. doi: 10.1093/hmg/2.8.1201. [DOI] [PubMed] [Google Scholar]
- Homma S., Gapstur S. M., Coffey A., Valtin H., Dousa T. P. Role of cAMP-phosphodiesterase isozymes in pathogenesis of murine nephrogenic diabetes insipidus. Am J Physiol. 1991 Aug;261(2 Pt 2):F345–F353. doi: 10.1152/ajprenal.1991.261.2.F345. [DOI] [PubMed] [Google Scholar]
- Jans D. A., van Oost B. A., Ropers H. H., Fahrenholz F. Derivatives of somatic cell hybrids which carry the human gene locus for nephrogenic diabetes insipidus (NDI) express functional vasopressin renal V2-type receptors. J Biol Chem. 1990 Sep 15;265(26):15379–15382. [PubMed] [Google Scholar]
- Kambouris M., Dlouhy S. R., Trofatter J. A., Conneally P. M., Hodes M. E. Localization of the gene for X-linked nephrogenic diabetes insipidus to Xq28. Am J Med Genet. 1988 Jan;29(1):239–246. doi: 10.1002/ajmg.1320290138. [DOI] [PubMed] [Google Scholar]
- Kawata R., Ohba Y., Yamamoto K., Miyaji T., Makita R., Ohga K., Watanabe S., Miwa S. Hyperunstable hemoglobin Koriyama anti-Hb Gun Hill insertion of five residues in the beta chain. Hemoglobin. 1988;12(4):311–321. doi: 10.3109/03630268808998032. [DOI] [PubMed] [Google Scholar]
- Knoers N., van der Heyden H., van Oost B. A., Ropers H. H., Monnens L., Willems J. Nephrogenic diabetes insipidus: close linkage with markers from the distal long arm of the human X chromosome. Hum Genet. 1988 Sep;80(1):31–38. doi: 10.1007/BF00451451. [DOI] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Ketterling R. P., Bridge P. J., Lillicrap D. P., Sommer S. S. Mutations causing hemophilia B: direct estimate of the underlying rates of spontaneous germ-line transitions, transversions, and deletions in a human gene. Am J Hum Genet. 1990 Aug;47(2):202–217. [PMC free article] [PubMed] [Google Scholar]
- Kornreich R., Bishop D. F., Desnick R. J. Alpha-galactosidase A gene rearrangements causing Fabry disease. Identification of short direct repeats at breakpoints in an Alu-rich gene. J Biol Chem. 1990 Jun 5;265(16):9319–9326. [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Cooper D. N. Gene deletions causing human genetic disease: mechanisms of mutagenesis and the role of the local DNA sequence environment. Hum Genet. 1991 Mar;86(5):425–441. doi: 10.1007/BF00194629. [DOI] [PubMed] [Google Scholar]
- Kremer H., Mariman E., Otten B. J., Moll G. W., Jr, Stoelinga G. B., Wit J. M., Jansen M., Drop S. L., Faas B., Ropers H. H. Cosegregation of missense mutations of the luteinizing hormone receptor gene with familial male-limited precocious puberty. Hum Mol Genet. 1993 Nov;2(11):1779–1783. doi: 10.1093/hmg/2.11.1779. [DOI] [PubMed] [Google Scholar]
- Kuhl D. P., Caskey C. T. Trinucleotide repeats and genome variation. Curr Opin Genet Dev. 1993 Jun;3(3):404–407. doi: 10.1016/0959-437x(93)90112-3. [DOI] [PubMed] [Google Scholar]
- Langley J. M., Balfe J. W., Selander T., Ray P. N., Clarke J. T. Autosomal recessive inheritance of vasopressin-resistant diabetes insipidus. Am J Med Genet. 1991 Jan;38(1):90–94. doi: 10.1002/ajmg.1320380120. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., O'Carroll A. M., McBride O. W., Konig M., Morel A., Brownstein M. J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992 May 28;357(6376):336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- Merendino J. J., Jr, Speigel A. M., Crawford J. D., O'Carroll A. M., Brownstein M. J., Lolait S. J. Brief report: a mutation in the vasopressin V2-receptor gene in a kindred with X-linked nephrogenic diabetes insipidus. N Engl J Med. 1993 May 27;328(21):1538–1541. doi: 10.1056/NEJM199305273282106. [DOI] [PubMed] [Google Scholar]
- Mita S., Rizzuto R., Moraes C. T., Shanske S., Arnaudo E., Fabrizi G. M., Koga Y., DiMauro S., Schon E. A. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res. 1990 Feb 11;18(3):561–567. doi: 10.1093/nar/18.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses A. M., Miller J. L., Levine M. A. Two distinct pathophysiological mechanisms in congenital nephrogenic diabetes insipidus. J Clin Endocrinol Metab. 1988 Jun;66(6):1259–1264. doi: 10.1210/jcem-66-6-1259. [DOI] [PubMed] [Google Scholar]
- Nathans J. Rhodopsin: structure, function, and genetics. Biochemistry. 1992 Jun 2;31(21):4923–4931. doi: 10.1021/bi00136a001. [DOI] [PubMed] [Google Scholar]
- Pan Y., Metzenberg A., Das S., Jing B., Gitschier J. Mutations in the V2 vasopressin receptor gene are associated with X-linked nephrogenic diabetes insipidus. Nat Genet. 1992 Oct;2(2):103–106. doi: 10.1038/ng1092-103. [DOI] [PubMed] [Google Scholar]
- Parma J., Duprez L., Van Sande J., Cochaux P., Gervy C., Mockel J., Dumont J., Vassart G. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993 Oct 14;365(6447):649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- Pollak M. R., Brown E. M., Chou Y. H., Hebert S. C., Marx S. J., Steinmann B., Levi T., Seidman C. E., Seidman J. G. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993 Dec 31;75(7):1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- Richards A. J., Lloyd J. C., Narcisi P., Ward P. N., Nicholls A. C., De Paepe A., Pope F. M. A 27-bp deletion from one allele of the type III collagen gene (COL3A1) in a large family with Ehlers-Danlos syndrome type IV. Hum Genet. 1992 Jan;88(3):325–330. doi: 10.1007/BF00197268. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., Cowley G. S., McGee T. L., Sandberg M. A., Berson E. L., Dryja T. P. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat Genet. 1992 Jun;1(3):209–213. doi: 10.1038/ng0692-209. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M., Chasin L. A. Isolation of mammalian cell mutants deficient in glucose-6-phosphate dehydrogenase activity: linkage to hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):493–497. doi: 10.1073/pnas.72.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal W., Antaramian A., Gilbert S., Birnbaumer M. Nephrogenic diabetes insipidus. A V2 vasopressin receptor unable to stimulate adenylyl cyclase. J Biol Chem. 1993 Jun 25;268(18):13030–13033. [PubMed] [Google Scholar]
- Rosenthal W., Seibold A., Antaramian A., Lonergan M., Arthus M. F., Hendy G. N., Birnbaumer M., Bichet D. G. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature. 1992 Sep 17;359(6392):233–235. doi: 10.1038/359233a0. [DOI] [PubMed] [Google Scholar]
- Rubnitz J., Subramani S. The minimum amount of homology required for homologous recombination in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2253–2258. doi: 10.1128/mcb.4.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese T. M., Fraser C. M. In vitro mutagenesis and the search for structure-function relationships among G protein-coupled receptors. Biochem J. 1992 Apr 1;283(Pt 1):1–19. doi: 10.1042/bj2830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold A., Brabet P., Rosenthal W., Birnbaumer M. Structure and chromosomal localization of the human antidiuretic hormone receptor gene. Am J Hum Genet. 1992 Nov;51(5):1078–1083. [PMC free article] [PubMed] [Google Scholar]
- Shenker A., Laue L., Kosugi S., Merendino J. J., Jr, Minegishi T., Cutler G. B., Jr A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993 Oct 14;365(6447):652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Tanoue A., Endo F., Akaboshi I., Oono T., Arata J., Matsuda I. Molecular defect in siblings with prolidase deficiency and absence or presence of clinical symptoms. A 0.8-kb deletion with breakpoints at the short, direct repeat in the PEPD gene and synthesis of abnormal messenger RNA and inactive polypeptide. J Clin Invest. 1991 Apr;87(4):1171–1176. doi: 10.1172/JCI115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C., Arai K., Hung W., Chrousos G. P. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J Clin Invest. 1993 Nov;92(5):2458–2461. doi: 10.1172/JCI116853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajcman H., Blouquit Y., Vasseur C., Le Querrec A., Laniece M., Melevendi C., Rasore A., Galacteros F. Two new human hemoglobin variants caused by unusual mutational events: Hb Zaïre contains a five residue repetition within the alpha-chain and Hb Duino has two residues substituted in the beta-chain. Hum Genet. 1992 Aug;89(6):676–680. doi: 10.1007/BF00221961. [DOI] [PubMed] [Google Scholar]
- Wess J. Mutational analysis of muscarinic acetylcholine receptors: structural basis of ligand/receptor/G protein interactions. Life Sci. 1993;53(19):1447–1463. doi: 10.1016/0024-3205(93)90618-d. [DOI] [PubMed] [Google Scholar]
- van den Ouweland A. M., Dreesen J. C., Verdijk M., Knoers N. V., Monnens L. A., Rocchi M., van Oost B. A. Mutations in the vasopressin type 2 receptor gene (AVPR2) associated with nephrogenic diabetes insipidus. Nat Genet. 1992 Oct;2(2):99–102. doi: 10.1038/ng1092-99. [DOI] [PubMed] [Google Scholar]





