Abstract
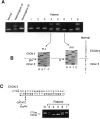
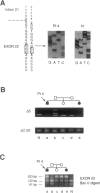
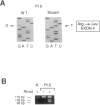
Phosphofructokinase (PFK) catalyzes the rate-limiting step of glycolysis. Deficiency of the muscle enzyme is manifested by exercise intolerance and a compensated hemolytic anemia. Case reports of this autosomal recessive disease suggest a predominance in Ashkenazi Jews in the United States. We have explored the genetic basis for this illness in nine affected families and surveyed the normal Ashkenazi population for the mutations we have found. Genomic DNA was amplified using PCR, and denaturing gradient-gel electrophoresis was used to localize exons with possible mutations. The polymorphic exons were sequenced or digested with restriction enzymes. A previously described splicing mutation, delta 5, accounted for 11 (61%) of 18 abnormal alleles in the nine families. A single base deletion leading to a frameshift mutation in exon 22 (delta C-22) was found in six of seven alleles. A third mutation, resulting in a nonconservative amino acid substitution in exon 4, accounted for the remaining allele. Thus, three mutations could account for all illness in this group, and two mutations could account for 17 of 18 alleles. In screening 250 normal Ashkenazi individuals for all three mutations, we found only one delta 5 allele. Clinical data revealed no correlation between the particular mutations and symptoms, but male patients were more symptomatic than females, and only males had frank hemolysis and hyperuricemia. Because PFK deficiency in Ashkenazi Jews is caused by a limited number of mutations, screening genomic DNA from peripheral blood for the described mutations in this population should enable rapid diagnosis without muscle biopsy.
Full text
PDF

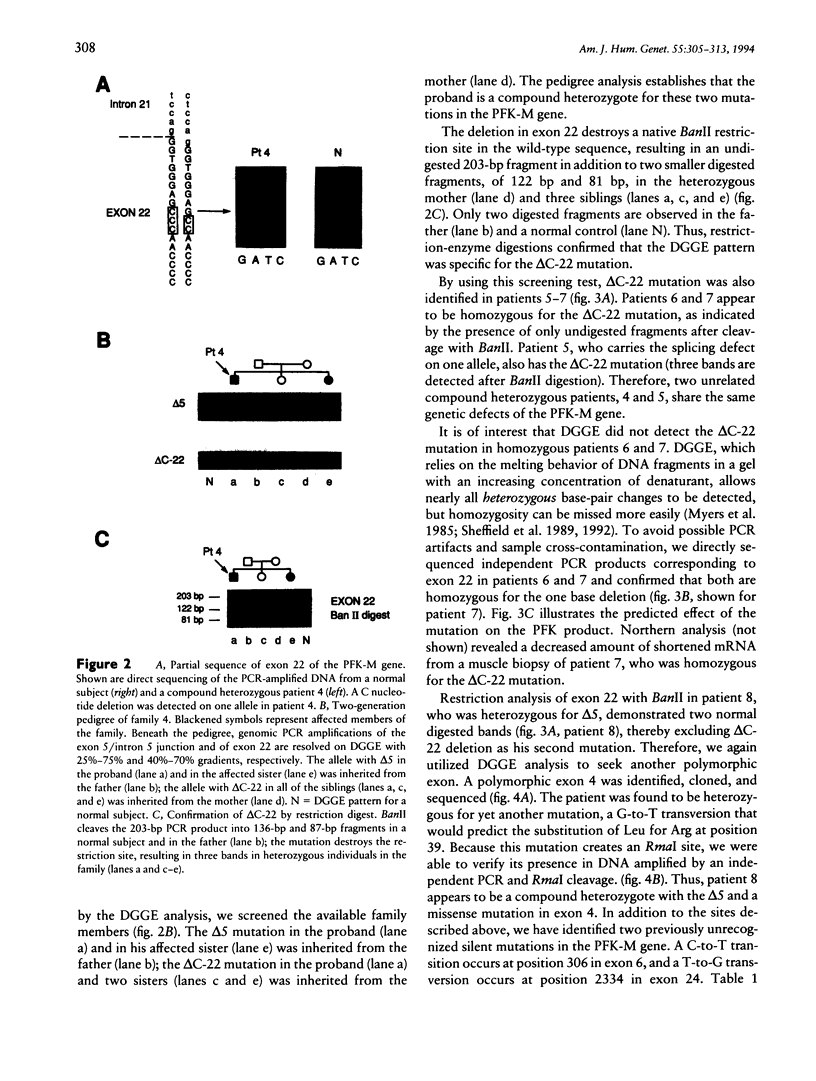
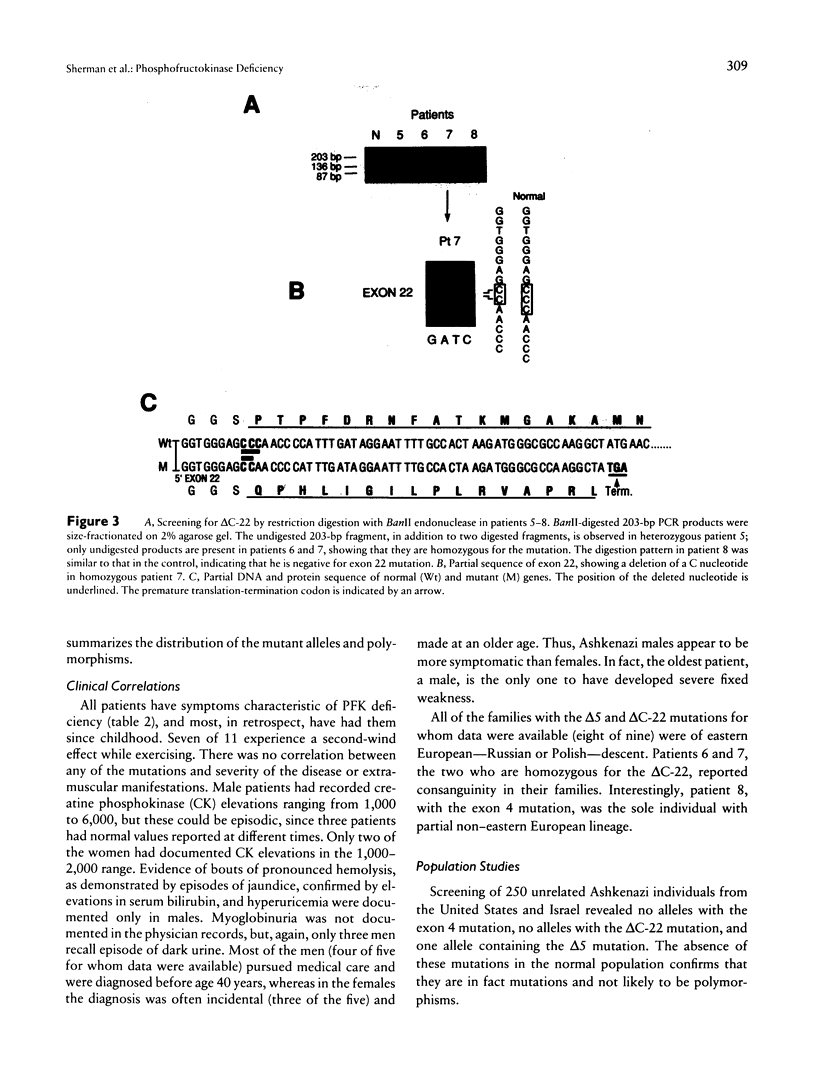
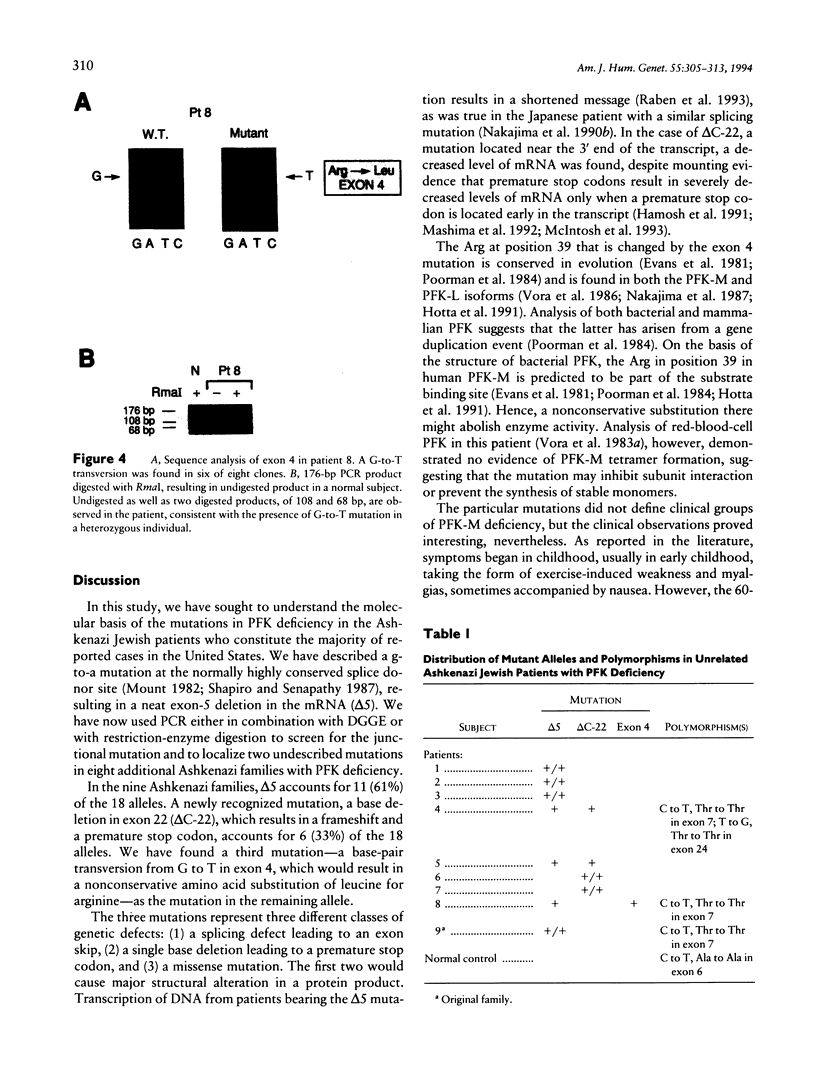
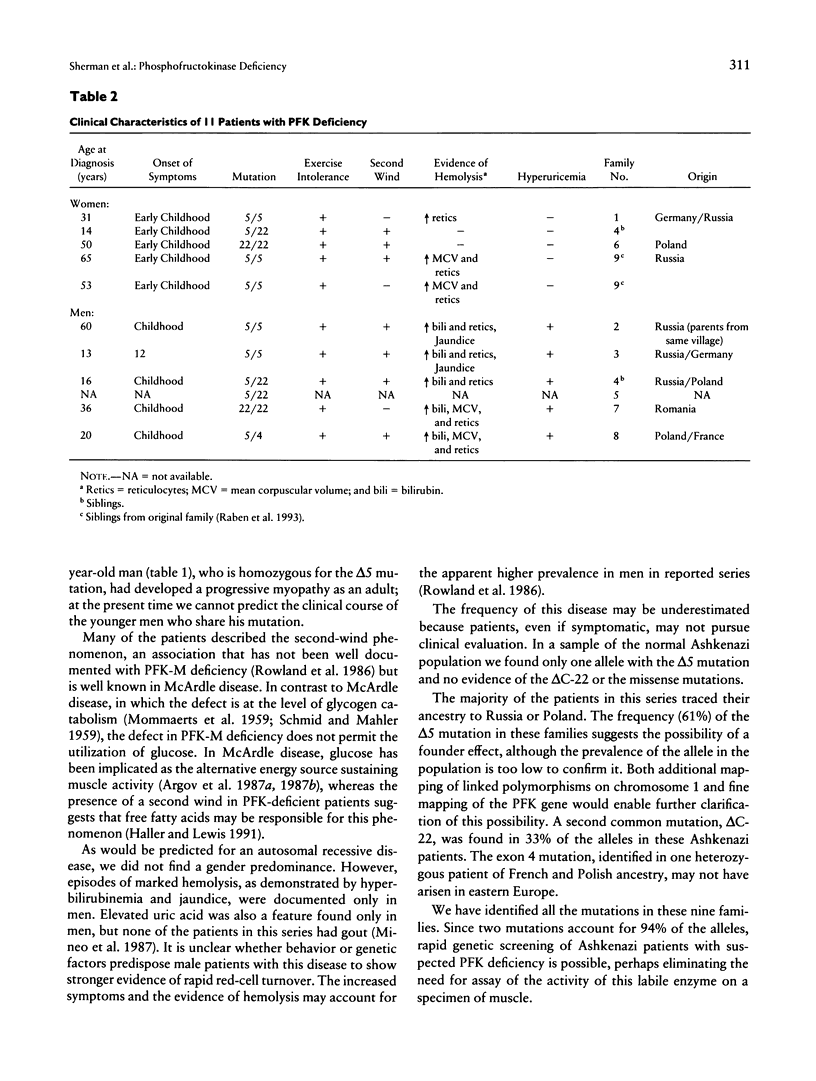


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argov Z., Bank W. J., Maris J., Chance B. Muscle energy metabolism in McArdle's syndrome by in vivo phosphorus magnetic resonance spectroscopy. Neurology. 1987 Nov;37(11):1720–1724. doi: 10.1212/wnl.37.11.1720. [DOI] [PubMed] [Google Scholar]
- Argov Z., Maris J., Damico L., Koruda M., Roth Z., Leigh J. S., Jr, Chance B. Continuous, graded steady-state muscle work in rats studied by in vivo 31P-NMR. J Appl Physiol (1985) 1987 Oct;63(4):1428–1433. doi: 10.1152/jappl.1987.63.4.1428. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Evans P. R., Farrants G. W., Hudson P. J. Phosphofructokinase: structure and control. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):53–62. doi: 10.1098/rstb.1981.0059. [DOI] [PubMed] [Google Scholar]
- Haller R. G., Lewis S. F. Glucose-induced exertional fatigue in muscle phosphofructokinase deficiency. N Engl J Med. 1991 Feb 7;324(6):364–369. doi: 10.1056/NEJM199102073240603. [DOI] [PubMed] [Google Scholar]
- Hamosh A., Trapnell B. C., Zeitlin P. L., Montrose-Rafizadeh C., Rosenstein B. J., Crystal R. G., Cutting G. R. Severe deficiency of cystic fibrosis transmembrane conductance regulator messenger RNA carrying nonsense mutations R553X and W1316X in respiratory epithelial cells of patients with cystic fibrosis. J Clin Invest. 1991 Dec;88(6):1880–1885. doi: 10.1172/JCI115510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K., Nakajima H., Yamasaki T., Hamaguchi T., Kuwajima M., Noguchi T., Tanaka T., Kono N., Tarui S. Rat-liver-type phosphofructokinase mRNA. Structure, tissue distribution and regulation. Eur J Biochem. 1991 Dec 5;202(2):293–298. doi: 10.1111/j.1432-1033.1991.tb16375.x. [DOI] [PubMed] [Google Scholar]
- Layzer R. B., Rowland L. P., Ranney H. M. Muscle phosphofructokinase deficiency. Arch Neurol. 1967 Nov;17(5):512–523. doi: 10.1001/archneur.1967.00470290066009. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Vora S., Haller R. G. Abnormal oxidative metabolism and O2 transport in muscle phosphofructokinase deficiency. J Appl Physiol (1985) 1991 Jan;70(1):391–398. doi: 10.1152/jappl.1991.70.1.391. [DOI] [PubMed] [Google Scholar]
- Mashima Y., Murakami A., Weleber R. G., Kennaway N. G., Clarke L., Shiono T., Inana G. Nonsense-codon mutations of the ornithine aminotransferase gene with decreased levels of mutant mRNA in gyrate atrophy. Am J Hum Genet. 1992 Jul;51(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- McIntosh I., Hamosh A., Dietz H. C. Nonsense mutations and diminished mRNA levels. Nat Genet. 1993 Jul;4(3):219–219. doi: 10.1038/ng0793-219. [DOI] [PubMed] [Google Scholar]
- Mineo I., Kono N., Hara N., Shimizu T., Yamada Y., Kawachi M., Kiyokawa H., Wang Y. L., Tarui S. Myogenic hyperuricemia. A common pathophysiologic feature of glycogenosis types III, V, and VII. N Engl J Med. 1987 Jul 9;317(2):75–80. doi: 10.1056/NEJM198707093170203. [DOI] [PubMed] [Google Scholar]
- Mommaerts W. F., Illingworth B., Pearson C. M., Guillory R. J., Seraydarian K. A FUNCTIONAL DISORDER OF MUSCLE ASSOCIATED WITH THE ABSENCE OF PHOSPHORYLASE. Proc Natl Acad Sci U S A. 1959 Jun;45(6):791–797. doi: 10.1073/pnas.45.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Kono N., Yamasaki T., Hamaguchi T., Hotta K., Kuwajima M., Noguchi T., Tanaka T., Tarui S. Tissue specificity in expression and alternative RNA splicing of human phosphofructokinase-M and -L genes. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1317–1321. doi: 10.1016/s0006-291x(05)80931-3. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Kono N., Yamasaki T., Hotta K., Kawachi M., Kuwajima M., Noguchi T., Tanaka T., Tarui S. Genetic defect in muscle phosphofructokinase deficiency. Abnormal splicing of the muscle phosphofructokinase gene due to a point mutation at the 5'-splice site. J Biol Chem. 1990 Jun 5;265(16):9392–9395. [PubMed] [Google Scholar]
- Nakajima H., Noguchi T., Yamasaki T., Kono N., Tanaka T., Tarui S. Cloning of human muscle phosphofructokinase cDNA. FEBS Lett. 1987 Oct 19;223(1):113–116. doi: 10.1016/0014-5793(87)80519-7. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Yamasaki T., Noguchi T., Tanaka T., Kono N., Tarui S. Evidence for alternative RNA splicing and possible alternative promoters in the human muscle phosphofructokinase gene at the 5' untranslated region. Biochem Biophys Res Commun. 1990 Jan 30;166(2):637–641. doi: 10.1016/0006-291x(90)90856-i. [DOI] [PubMed] [Google Scholar]
- Poorman R. A., Randolph A., Kemp R. G., Heinrikson R. L. Evolution of phosphofructokinase--gene duplication and creation of new effector sites. 1984 May 31-Jun 6Nature. 309(5967):467–469. doi: 10.1038/309467a0. [DOI] [PubMed] [Google Scholar]
- Raben N., Barbetti F., Cama A., Lesniak M. A., Lillioja S., Zimmet P., Serjeantson S. W., Taylor S. I., Roth J. Normal coding sequence of insulin gene in Pima Indians and Nauruans, two groups with highest prevalence of type II diabetes. Diabetes. 1991 Jan;40(1):118–122. doi: 10.2337/diab.40.1.118. [DOI] [PubMed] [Google Scholar]
- Raben N., Sherman J., Miller F., Mena H., Plotz P. A 5' splice junction mutation leading to exon deletion in an Ashkenazic Jewish family with phosphofructokinase deficiency (Tarui disease). J Biol Chem. 1993 Mar 5;268(7):4963–4967. [PubMed] [Google Scholar]
- SCHMID R., MAHLER R. Chronic progressive myopathy with myoglobinuria: demonstration of a glycogenolytic defect in the muscle. J Clin Invest. 1959 Nov;38:2044–2058. doi: 10.1172/JCI103983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P. M., Reddy G. R., Babior B. M., McLachlan A. Alternative splicing of the transcript encoding the human muscle isoenzyme of phosphofructokinase. J Biol Chem. 1990 Jun 5;265(16):9006–9010. [PubMed] [Google Scholar]
- Sharma P. M., Reddy G. R., Vora S., Babior B. M., McLachlan A. Cloning and expression of a human muscle phosphofructokinase cDNA. Gene. 1989 Apr 15;77(1):177–183. doi: 10.1016/0378-1119(89)90372-7. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Beck J. S., Nichols B., Cousineau A., Lidral A. C., Stone E. M. Detection of multiallele polymorphisms within gene sequences by GC-clamped denaturing gradient gel electrophoresis. Am J Hum Genet. 1992 Mar;50(3):567–575. [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARUI S., OKUNO G., IKURA Y., TANAKA T., SUDA M., NISHIKAWA M. PHOSPHOFRUCTOKINASE DEFICIENCY IN SKELETAL MUSCLE. A NEW TYPE OF GLYCOGENOSIS. Biochem Biophys Res Commun. 1965 May 3;19:517–523. doi: 10.1016/0006-291x(65)90156-7. [DOI] [PubMed] [Google Scholar]
- Valdez B. C., Chen Z., Sosa M. G., Younathan E. S., Chang S. H. Human 6-phosphofructo-1-kinase gene has an additional intron upstream of start codon. Gene. 1989 Mar 15;76(1):167–169. doi: 10.1016/0378-1119(89)90019-x. [DOI] [PubMed] [Google Scholar]
- Van Keuren M., Drabkin H., Hart I., Harker D., Patterson D., Vora S. Regional assignment of human liver-type 6-phosphofructokinase to chromosome 21q22.3 by using somatic cell hybrids and a monoclonal anti-L antibody. Hum Genet. 1986 Sep;74(1):34–40. doi: 10.1007/BF00278782. [DOI] [PubMed] [Google Scholar]
- Vora S., Davidson M., Seaman C., Miranda A. F., Noble N. A., Tanaka K. R., Frenkel E. P., Dimauro S. Heterogeneity of the molecular lesions in inherited phosphofructokinase deficiency. J Clin Invest. 1983 Dec;72(6):1995–2006. doi: 10.1172/JCI111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S., Durham S., de Martinville B., George D. L., Francke U. Assignment of the human gene for muscle-type phosphofructokinase (PFKM) to chromosome 1 (region cen leads to q32) using somatic cell hybrids and monoclonal anti-M antibody. Somatic Cell Genet. 1982 Jan;8(1):95–104. doi: 10.1007/BF01538653. [DOI] [PubMed] [Google Scholar]
- Vora S., Hong F., Olender E. Isolation of a cDNA for human muscle 6-phosphofructokinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):615–621. doi: 10.1016/0006-291x(86)90037-9. [DOI] [PubMed] [Google Scholar]
- Vora S. Isozymes of human phosphofructokinase: biochemical and genetic aspects. Isozymes Curr Top Biol Med Res. 1983;11:3–23. [PubMed] [Google Scholar]
- Vora S., Miranda A. F., Hernandez E., Francke U. Regional assignment of the human gene for platelet-type phosphofructokinase (PFKP) to chromosome 10p: novel use of polyspecific rodent antisera to localize human enzyme genes. Hum Genet. 1983;63(4):374–379. doi: 10.1007/BF00274765. [DOI] [PubMed] [Google Scholar]
- Yamasaki T., Nakajima H., Kono N., Hotta K., Yamada K., Imai E., Kuwajima M., Noguchi T., Tanaka T., Tarui S. Structure of the entire human muscle phosphofructokinase-encoding gene: a two-promoter system. Gene. 1991 Aug 15;104(2):277–282. doi: 10.1016/0378-1119(91)90262-a. [DOI] [PubMed] [Google Scholar]