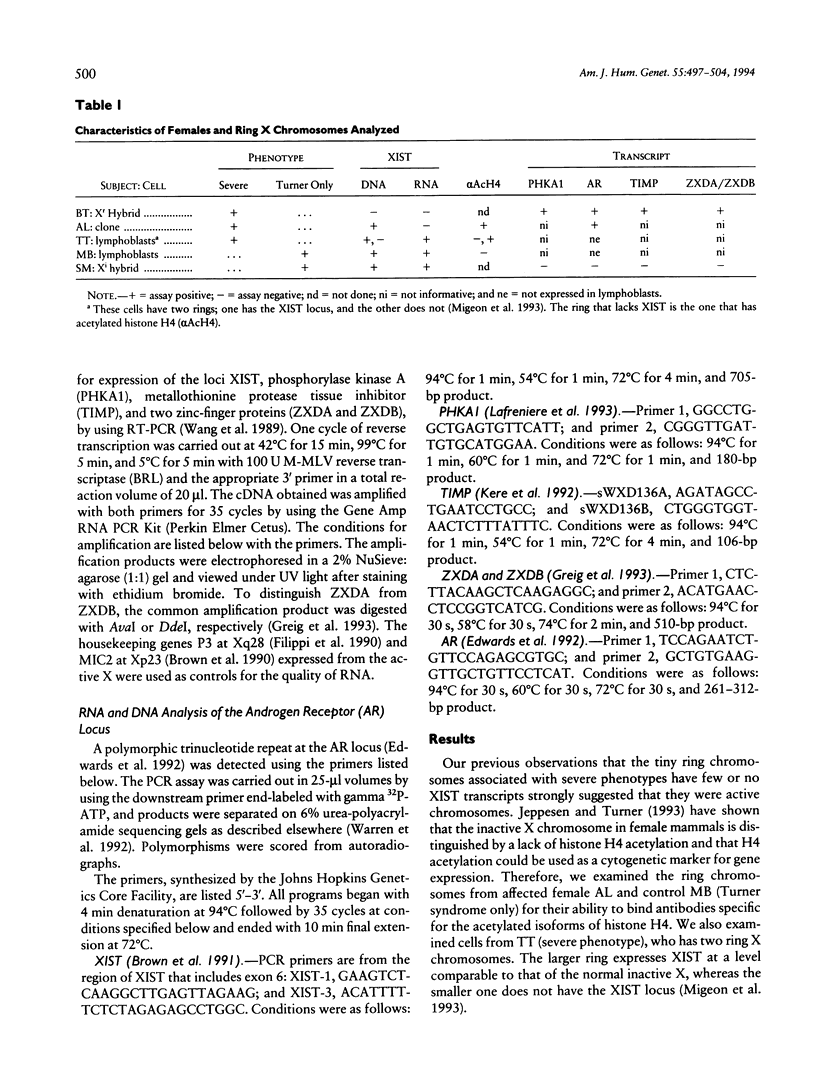
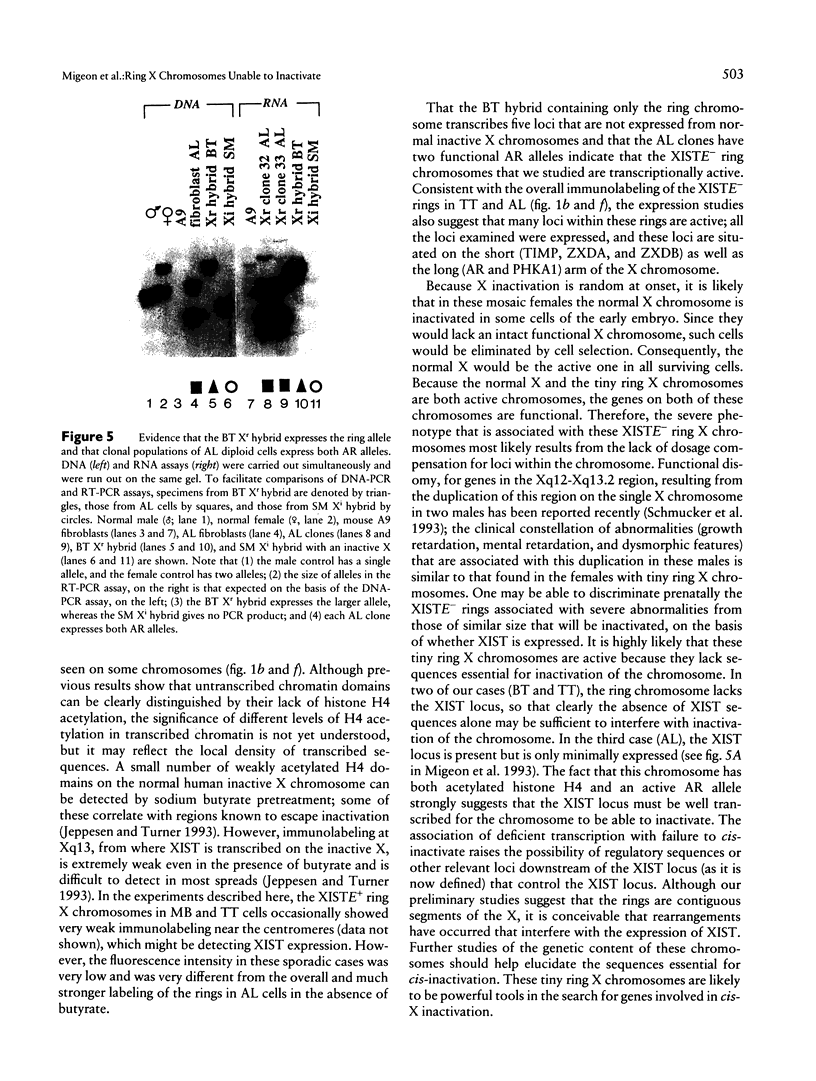
Abstract
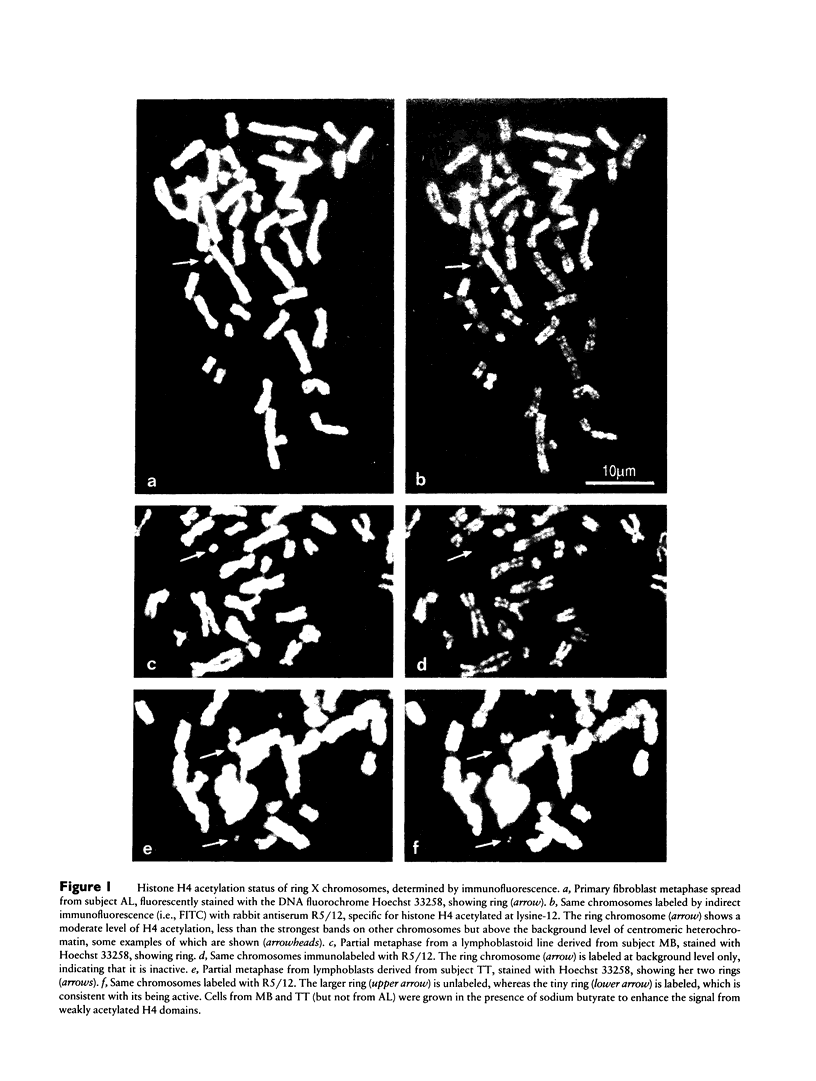
Mental retardation and a constellation of congenital malformations not usually associated with Turner syndrome are seen in some females with a mosaic 45,X/46,X,r(X) karyotype. Studies of these females show that the XIST locus on their tiny ring X chromosomes is either not present or not expressed. As XIST transcription is well correlated with inactivation of the X chromosome in female somatic cells and spermatogonia, nonexpression of the locus even when it is present suggests that these chromosomes are transcriptionally active. We examined the transcriptional activity of ring X chromosomes lacking XIST expression (XISTE-), from three females with severe phenotypes. The two tiny ring X chromosomes studied with an antibody specific for the acetylated isoforms of histone H4 marking transcribed chromatin domains were labeled at a level consistent with their being active. We also examined tow of the XISTE- ring chromosomes to determine whether genes that are normally silent on an inactive X are expressed from these chromosomes. Analyses of hybrid cells show that TIMP, ZXDA, and ZXDB loci on the proximal short arm, and AR and PHKA1 loci on the long arm, are well expressed from the tiny ring X chromosome lacking XIST DNA. Studies of the ring chromosome that has XIST DNA but does not transcribe it show that its AR allele is transcribed along with the one on the normal X allele.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballabio A., Willard H. F. Mammalian X-chromosome inactivation and the XIST gene. Curr Opin Genet Dev. 1992 Jun;2(3):439–447. doi: 10.1016/s0959-437x(05)80155-8. [DOI] [PubMed] [Google Scholar]
- Borsani G., Tonlorenzi R., Simmler M. C., Dandolo L., Arnaud D., Capra V., Grompe M., Pizzuti A., Muzny D., Lawrence C. Characterization of a murine gene expressed from the inactive X chromosome. Nature. 1991 May 23;351(6324):325–329. doi: 10.1038/351325a0. [DOI] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., Cooper P., Smith S., McCabe V. M., Norris D. P., Penny G. D., Patel D., Rastan S. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature. 1991 May 23;351(6324):329–331. doi: 10.1038/351329a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Ballabio A., Rupert J. L., Lafreniere R. G., Grompe M., Tonlorenzi R., Willard H. F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991 Jan 3;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Flenniken A. M., Williams B. R., Willard H. F. X chromosome inactivation of the human TIMP gene. Nucleic Acids Res. 1990 Jul 25;18(14):4191–4195. doi: 10.1093/nar/18.14.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Willard H. F. Noninactivation of a selectable human X-linked gene that complements a murine temperature-sensitive cell cycle defect. Am J Hum Genet. 1989 Oct;45(4):592–598. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dennis N. R., Collins A. L., Crolla J. A., Cockwell A. E., Fisher A. M., Jacobs P. A. Three patients with ring (X) chromosomes and a severe phenotype. J Med Genet. 1993 Jun;30(6):482–486. doi: 10.1136/jmg.30.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992 Feb;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Filippi M., Tribioli C., Toniolo D. Linkage and sequence conservation of the X-linked genes DXS253E (P3) and DXS254E (GdX) in mouse and man. Genomics. 1990 Jul;7(3):453–457. doi: 10.1016/0888-7543(90)90184-v. [DOI] [PubMed] [Google Scholar]
- Fraser N. J., Boyd Y., Brownlee G. G., Craig I. W. Multi-allelic RFLP for M27 beta, an anonymous single copy genomic clone at Xp11.3-Xcen [HGM9 provisional no. DXS255]. Nucleic Acids Res. 1987 Nov 25;15(22):9616–9616. doi: 10.1093/nar/15.22.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig G. M., Sharp C. B., Carrel L., Willard H. F. Duplicated zinc finger protein genes on the proximal short arm of the human X chromosome: isolation, characterization and X-inactivation studies. Hum Mol Genet. 1993 Oct;2(10):1611–1618. doi: 10.1093/hmg/2.10.1611. [DOI] [PubMed] [Google Scholar]
- Jeppesen P., Mitchell A., Turner B., Perry P. Antibodies to defined histone epitopes reveal variations in chromatin conformation and underacetylation of centric heterochromatin in human metaphase chromosomes. Chromosoma. 1992 Mar;101(5-6):322–332. doi: 10.1007/BF00346011. [DOI] [PubMed] [Google Scholar]
- Jeppesen P., Turner B. M. The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell. 1993 Jul 30;74(2):281–289. doi: 10.1016/0092-8674(93)90419-q. [DOI] [PubMed] [Google Scholar]
- Kere J., Nagaraja R., Mumm S., Ciccodicola A., D'Urso M., Schlessinger D. Mapping human chromosomes by walking with sequence-tagged sites from end fragments of yeast artificial chromosome inserts. Genomics. 1992 Oct;14(2):241–248. doi: 10.1016/s0888-7543(05)80212-5. [DOI] [PubMed] [Google Scholar]
- Kushnick T., Irons T. G., Wiley J. E., Gettig E. A., Rao K. W., Bowyer S. 45X/46X,r(X) with syndactyly and severe mental retardation. Am J Med Genet. 1987 Nov;28(3):567–574. doi: 10.1002/ajmg.1320280304. [DOI] [PubMed] [Google Scholar]
- Lafrenière R. G., Brown C. J., Rider S., Chelly J., Taillon-Miller P., Chinault A. C., Monaco A. P., Willard H. F. 2.6 Mb YAC contig of the human X inactivation center region in Xq13: physical linkage of the RPS4X, PHKA1, XIST and DXS128E genes. Hum Mol Genet. 1993 Aug;2(8):1105–1115. doi: 10.1093/hmg/2.8.1105. [DOI] [PubMed] [Google Scholar]
- Lindgren V., Chen C. P., Bryke C. R., Lichter P., Page D. C., Yang-Feng T. L. Cytogenetic and molecular characterization of marker chromosomes in patients with mosaic 45,X karyotypes. Hum Genet. 1992 Feb;88(4):393–398. doi: 10.1007/BF00215672. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- McCarrey J. R., Dilworth D. D. Expression of Xist in mouse germ cells correlates with X-chromosome inactivation. Nat Genet. 1992 Nov;2(3):200–203. doi: 10.1038/ng1192-200. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Luo S., Stasiowski B. A., Jani M., Axelman J., Van Dyke D. L., Weiss L., Jacobs P. A., Yang-Feng T. L., Wiley J. E. Deficient transcription of XIST from tiny ring X chromosomes in females with severe phenotypes. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12025–12029. doi: 10.1073/pnas.90.24.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler C., Soreq H., Wahrman J. X inactivation in mammalian testis is correlated with inactive X-specific transcription. Nat Genet. 1992 Nov;2(3):192–195. doi: 10.1038/ng1192-192. [DOI] [PubMed] [Google Scholar]
- Salido E. C., Yen P. H., Mohandas T. K., Shapiro L. J. Expression of the X-inactivation-associated gene XIST during spermatogenesis. Nat Genet. 1992 Nov;2(3):196–199. doi: 10.1038/ng1192-196. [DOI] [PubMed] [Google Scholar]
- Turner B. M., Fellows G. Specific antibodies reveal ordered and cell-cycle-related use of histone-H4 acetylation sites in mammalian cells. Eur J Biochem. 1989 Jan 15;179(1):131–139. doi: 10.1111/j.1432-1033.1989.tb14530.x. [DOI] [PubMed] [Google Scholar]
- Van Dyke D. L., Wiktor A., Palmer C. G., Miller D. A., Witt M., Babu V. R., Worsham M. J., Roberson J. R., Weiss L. Ullrich-Turner syndrome with a small ring X chromosome and presence of mental retardation. Am J Med Genet. 1992 Aug 1;43(6):996–1005. doi: 10.1002/ajmg.1320430617. [DOI] [PubMed] [Google Scholar]
- Van Dyke D. L., Wiktor A., Roberson J. R., Weiss L. Mental retardation in Turner syndrome. J Pediatr. 1991 Mar;118(3):415–417. doi: 10.1016/s0022-3476(05)82159-6. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren A. C., McInnis M. G., Blaschak J., Kaliatsidaki M., Petersen M. B., Chakravarti A., Antonarakis S. E. Dinucleotide repeat (GT)n markers on chromosome 21. Genomics. 1992 Nov;14(3):818–819. doi: 10.1016/s0888-7543(05)80198-3. [DOI] [PubMed] [Google Scholar]