Abstract
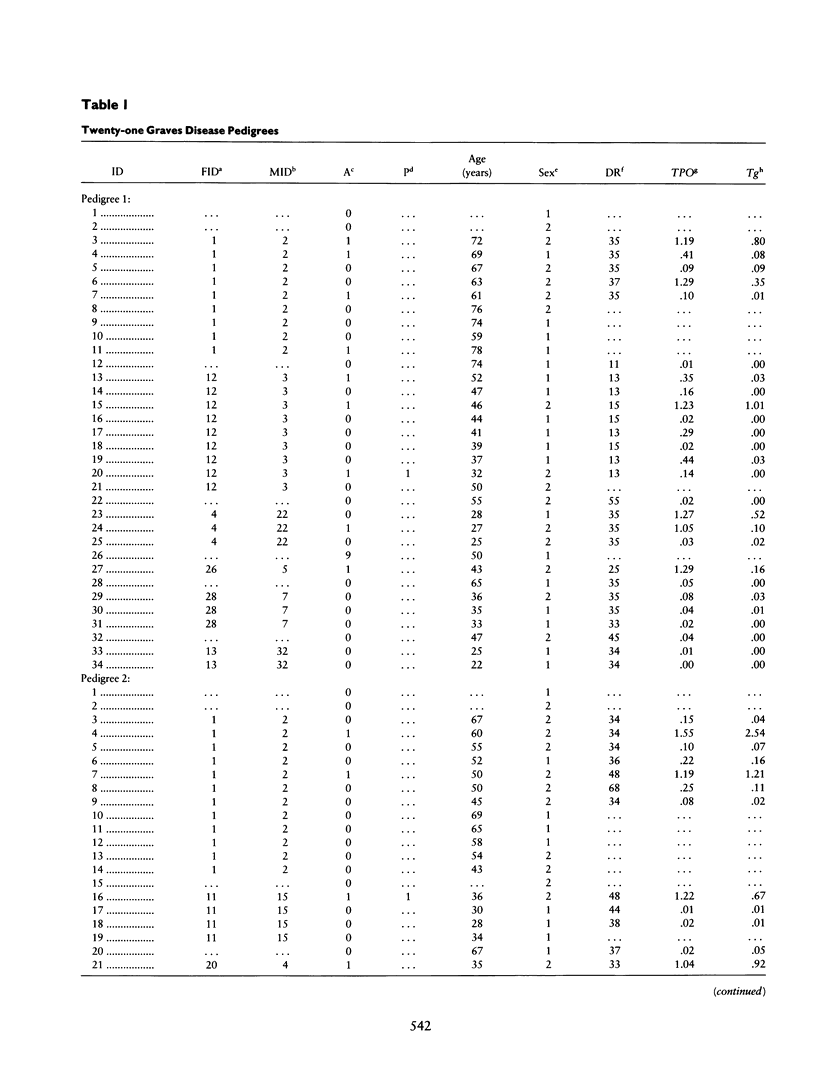
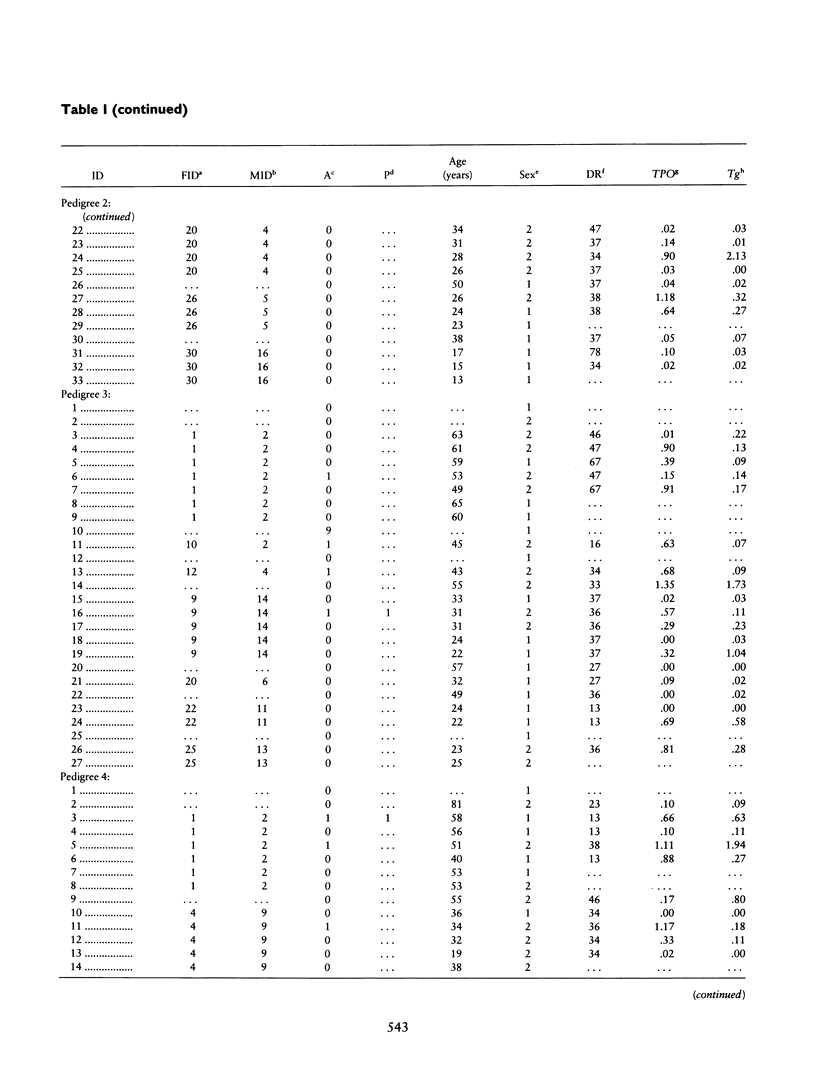
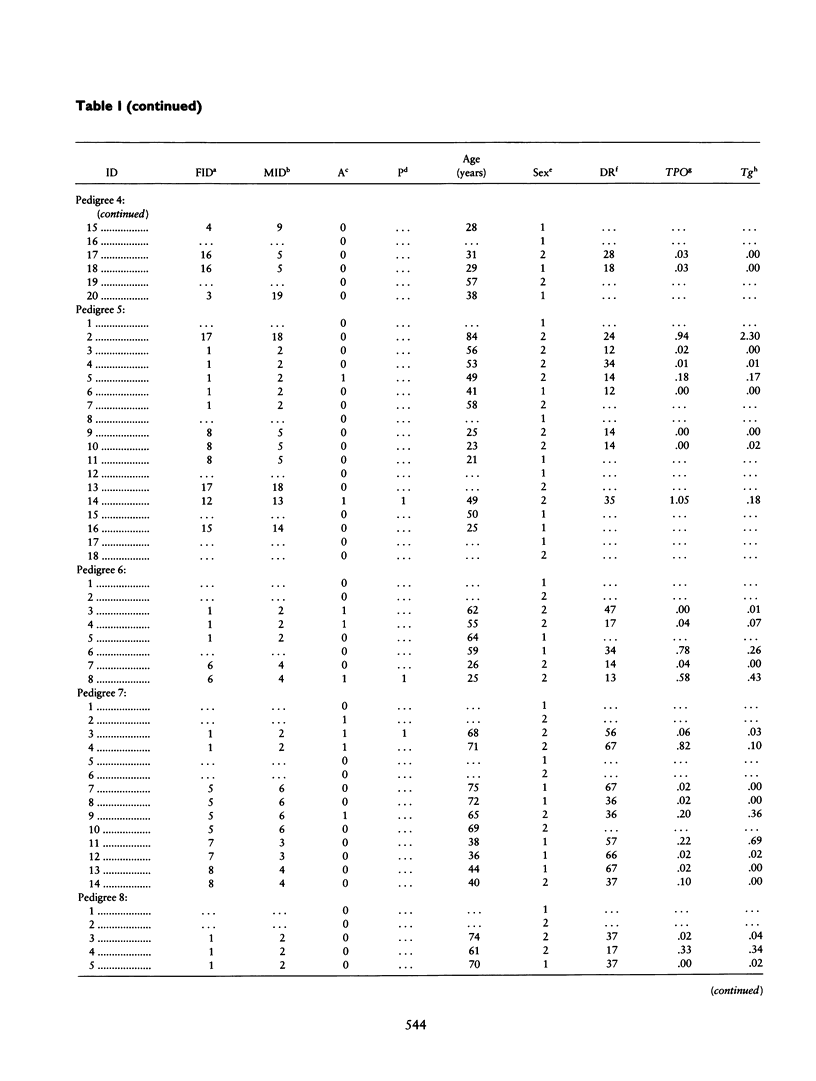
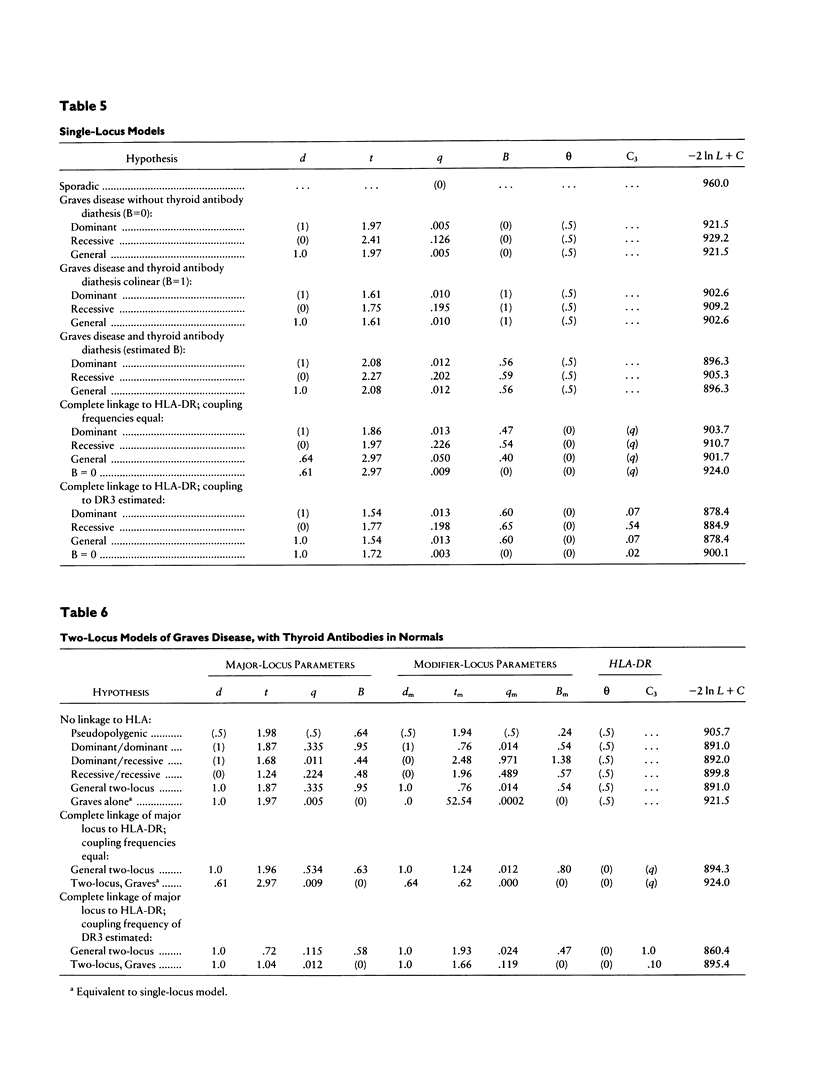
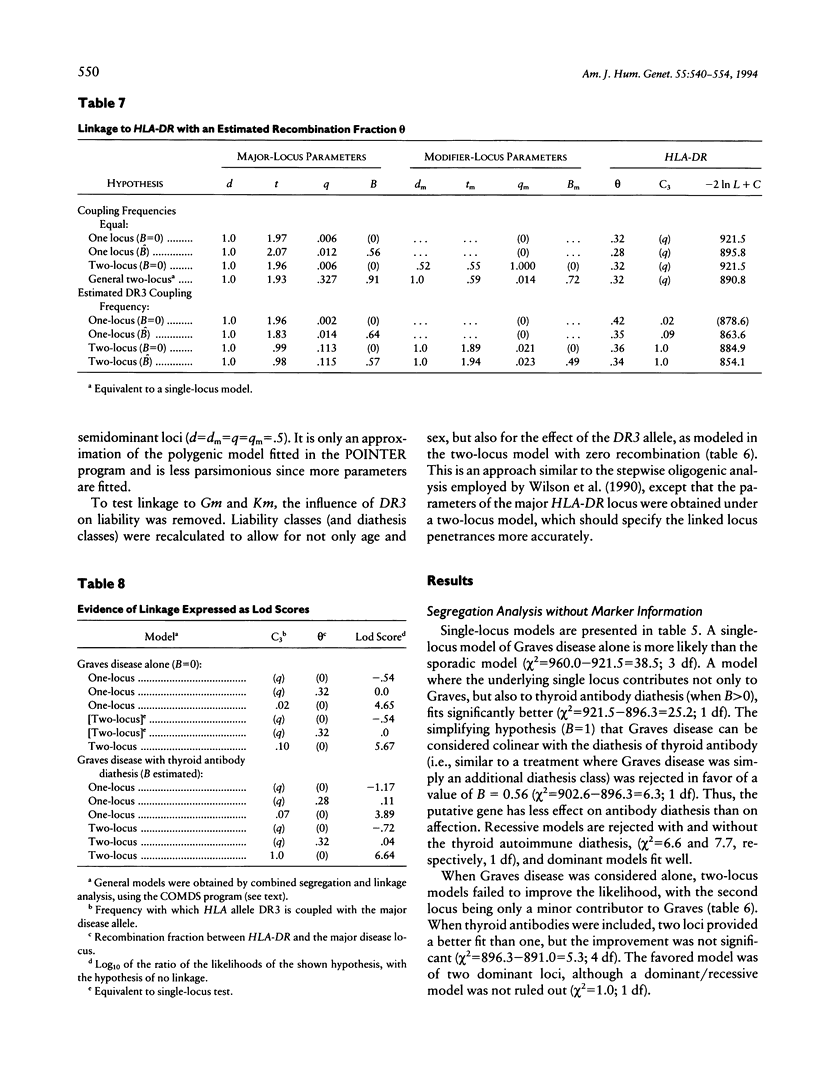
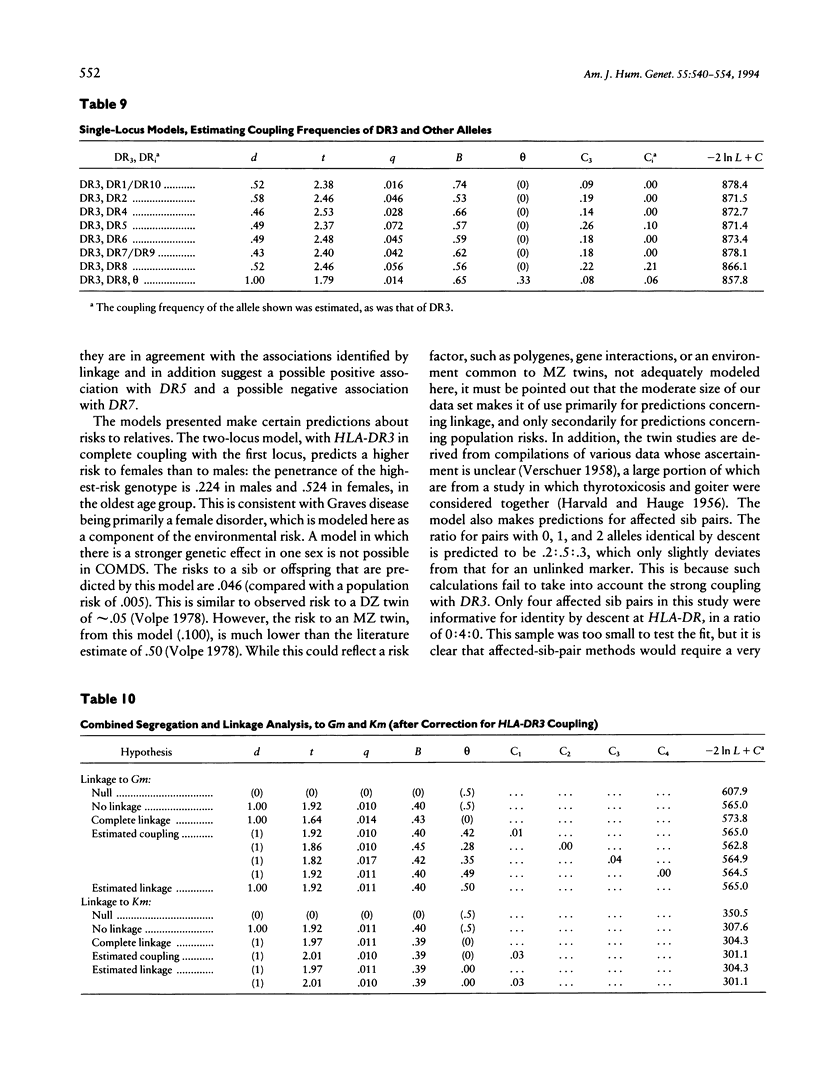
Combined segregation and linkage analysis is a powerful technique for modeling linkage to diseases whose etiology is more complex than the effect of a well-described single genetic locus and for investigating the influence of single genes on various aspects of the disease phenotype. Graves disease is familial and is associated with human leukocyte antigen (HLA) allele DR3. Probands with Graves disease, as well as close relatives, have raised levels of thyroid autoantibodies. This phenotypic information additional to affection status may be considered by the computer program COMDS for combined segregation and linkage analysis, when normals are classified into diathesis classes of increasing thyroid autoantibody titer. The ordinal model considers the cumulative odds of lying in successive classes, and a single additional parameter is introduced for each gene modeled. Distributional assumptions are avoided by providing estimates of the population frequencies of each class. Evidence for linkage was increased by considering the thyroid autoantibody diathesis and by testing two-locus models. The analysis revealed evidence for linkage to HLA-DR when the strong coupling of the linked locus to allele DR3 was considered (lod score of 6.6). Linkage analysis of the residual variation revealed no evidence of linkage to Gm, but a suggestion of linkage to Km.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allannic H., Fauchet R., Lorcy Y., Heim J., Gueguen M., Leguerrier A. M., Genetet B. HLA and Graves' disease: an association with HLA-DRw3. J Clin Endocrinol Metab. 1980 Oct;51(4):863–867. doi: 10.1210/jcem-51-4-863. [DOI] [PubMed] [Google Scholar]
- Armstrong B. G., Sloan M. Ordinal regression models for epidemiologic data. Am J Epidemiol. 1989 Jan;129(1):191–204. doi: 10.1093/oxfordjournals.aje.a115109. [DOI] [PubMed] [Google Scholar]
- Bidwell J. DNA-RFLP analysis and genotyping of HLA-DR and DQ antigens. Immunol Today. 1988 Jan;9(1):18–23. doi: 10.1016/0167-5699(88)91351-5. [DOI] [PubMed] [Google Scholar]
- Bonney G. E., Dunston G. M., Wilson J. Regressive logistic models for ordered and unordered polychotomous traits: application to affective disorders. Genet Epidemiol. 1989;6(1):211–215. doi: 10.1002/gepi.1370060137. [DOI] [PubMed] [Google Scholar]
- Bonney G. E., Lathrop G. M., Lalouel J. M. Combined linkage and segregation analysis using regressive models. Am J Hum Genet. 1988 Jul;43(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Skolnick M. H., Bishop D. T., Lee R. G., Burt R. W. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl J Med. 1988 Sep 1;319(9):533–537. doi: 10.1056/NEJM198809013190902. [DOI] [PubMed] [Google Scholar]
- Clerget-Darpoux F., Bonaïti-Pellié C., Hochez J. Effects of misspecifying genetic parameters in lod score analysis. Biometrics. 1986 Jun;42(2):393–399. [PubMed] [Google Scholar]
- Curtis D., Gurling H. M. Using a dummy quantitative variable to deal with multiple affection categories in genetic linkage analysis. Ann Hum Genet. 1991 Oct;55(Pt 4):321–327. doi: 10.1111/j.1469-1809.1991.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Demenais F., Lathrop M., Lalouel J. M. Robustness and power of the unified model in the analysis of quantitative measurements. Am J Hum Genet. 1986 Feb;38(2):228–234. [PMC free article] [PubMed] [Google Scholar]
- Farid N. R., Sampson L., Noel E. P., Barnard J. M., Mandeville R., Larsen B., Marshall W. H., Carter N. D. A study of human leukocyte D locus related antigens in Graves' disease. J Clin Invest. 1979 Jan;63(1):108–113. doi: 10.1172/JCI109263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid N. R. Understanding the genetics of autoimmune thyroid disease--still an illusive goal! J Clin Endocrinol Metab. 1992 Mar;74(3):495A–495B. doi: 10.1210/jcem.74.3.1740482. [DOI] [PubMed] [Google Scholar]
- HARVALD B., HAUGE M. A catamnestic investigation of Danish twins; a preliminary report. Dan Med Bull. 1956 Aug;3(5):150–158. [PubMed] [Google Scholar]
- Lalouel J. M., Le Mignon L., Simon M., Fauchet R., Bourel M., Rao D. C., Morton N. E. Genetic analysis of idiopathic hemochromatosis using both qualitative (disease status) and quantitative (serum iron) information. Am J Hum Genet. 1985 Jul;37(4):700–718. [PMC free article] [PubMed] [Google Scholar]
- Lalouel J. M., Morton N. E. Complex segregation analysis with pointers. Hum Hered. 1981;31(5):312–321. doi: 10.1159/000153231. [DOI] [PubMed] [Google Scholar]
- MacLean C. J., Morton N. E., Yee S. Combined analysis of genetic segregation and linkage under an oligogenic model. Comput Biomed Res. 1984 Oct;17(5):471–480. doi: 10.1016/0010-4809(84)90013-2. [DOI] [PubMed] [Google Scholar]
- Morton N. E. Analysis of family resemblance. I. Introduction. Am J Hum Genet. 1974 May;26(3):318–330. [PMC free article] [PubMed] [Google Scholar]
- Morton N. E., Shields D. C., Collins A. Genetic epidemiology of complex phenotypes. Ann Hum Genet. 1991 Oct;55(Pt 4):301–314. doi: 10.1111/j.1469-1809.1991.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Phillips D. I., Barker D. J., Rees Smith B., Didcote S., Morgan D. The geographical distribution of thyrotoxicosis in England according to the presence or absence of TSH-receptor antibodies. Clin Endocrinol (Oxf) 1985 Sep;23(3):283–287. doi: 10.1111/j.1365-2265.1985.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Ratanachaiyavong S., Gunn C. A., Bidwell E. A., Darke C., Hall R., McGregor A. M. DQA2 U allele: a genetic marker for relapse of Graves' disease. Clin Endocrinol (Oxf) 1990 Feb;32(2):241–251. doi: 10.1111/j.1365-2265.1990.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Ratanachaiyavong S., Lloyd L., Darke C., McGregor A. M. MHC-extended haplotypes in families of patients with Graves' disease. Hum Immunol. 1993 Feb;36(2):99–111. doi: 10.1016/0198-8859(93)90112-e. [DOI] [PubMed] [Google Scholar]
- Roman S. H., Greenberg D., Rubinstein P., Wallenstein S., Davies T. F. Genetics of autoimmune thyroid disease: lack of evidence for linkage to HLA within families. J Clin Endocrinol Metab. 1992 Mar;74(3):496–503. doi: 10.1210/jcem.74.3.1740483. [DOI] [PubMed] [Google Scholar]
- Skolnick M. H., Cannon-Albright L. A., Goldgar D. E., Ward J. H., Marshall C. J., Schumann G. B., Hogle H., McWhorter W. P., Wright E. C., Tran T. D. Inheritance of proliferative breast disease in breast cancer kindreds. Science. 1990 Dec 21;250(4988):1715–1720. doi: 10.1126/science.2270486. [DOI] [PubMed] [Google Scholar]
- Volpe R., Edmonds M., Lamki L., Clarke P. V., Row V. V. The pathogenesis of Graves' disease. A disorder of delayed hypersensitivity? Mayo Clin Proc. 1972 Nov;47(11):824–834. [PubMed] [Google Scholar]
- Weeks D. E., Lehner T., Squires-Wheeler E., Kaufmann C., Ott J. Measuring the inflation of the lod score due to its maximization over model parameter values in human linkage analysis. Genet Epidemiol. 1990;7(4):237–243. doi: 10.1002/gepi.1370070402. [DOI] [PubMed] [Google Scholar]
- Wilson A. F., Elston R. C., Sellers T. A., Bailey-Wilson J. E., Gersting J. M., Deen D. K., Sorant A. J., Tran L. D., Amos C. I., Siervogel R. M. Stepwise oligogenic segregation and linkage analysis illustrated with dopamine-beta-hydroxylase activity. Am J Med Genet. 1990 Mar;35(3):425–432. doi: 10.1002/ajmg.1320350321. [DOI] [PubMed] [Google Scholar]


