Abstract
We describe here the identification of defined mutations in both alleles of the fibrillin gene (FBN1) in a compound-heterozygote Marfan syndrome (MFS) child who had a very severe form of MFS resulting in death from cardiac failure at the age of 4 mo. The nonconsanguineous parents were both affected with MFS. The father's heterozygous point mutation has earlier been reported to result in W217G substitution, the mother was here shown to carry a heterozygous point mutation resulting in G2627R substitution, and the child had inherited both these mutations. The mutant FBN1 alleles were demonstrated to be transcribed with equal efficiency compared with the normal alleles, but metabolic labeling of fibroblast cultures from the child and both parents showed reduced biosynthesis and secretion of profibrillin. Also, the respective amounts of fibrillin in cell-culture media and extracellular-matrix extracts were markedly diminished, particularly in the cell cultures from father and child. In addition, immunofluorescence analysis of the cell cultures of all three family members revealed a drastically reduced amount of microfibrils, and virtually no visible fibrils could be seen in the case of the compound-heterozygote child. These findings demonstrate incomplete dominance of fibrillin mutations and underline the fatal consequences of the complete absence of normal fibrillin molecules in the microfibrils.
Full text
PDF


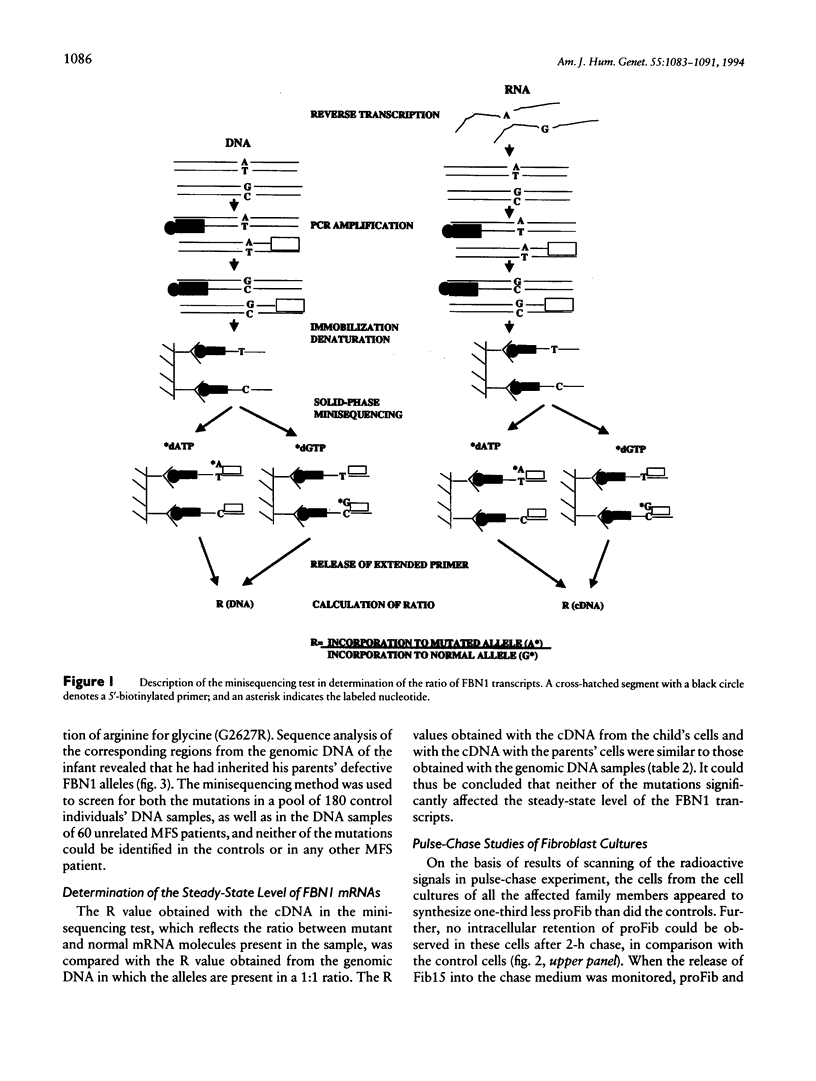
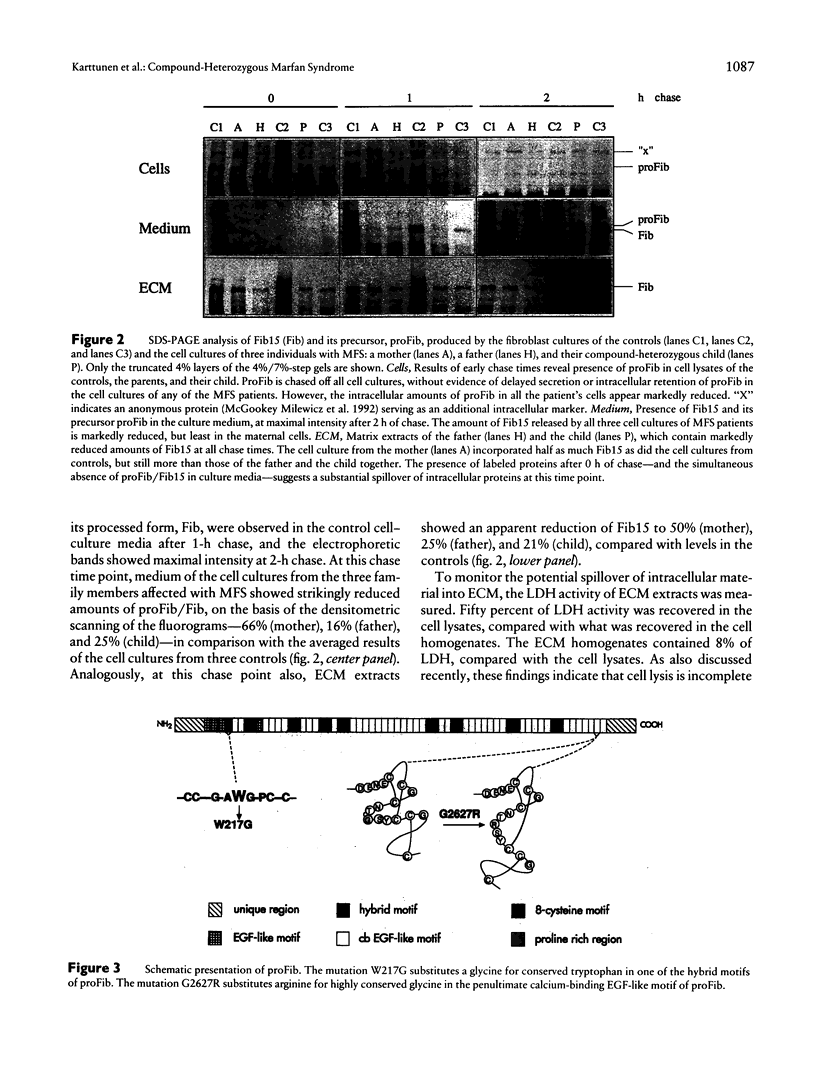

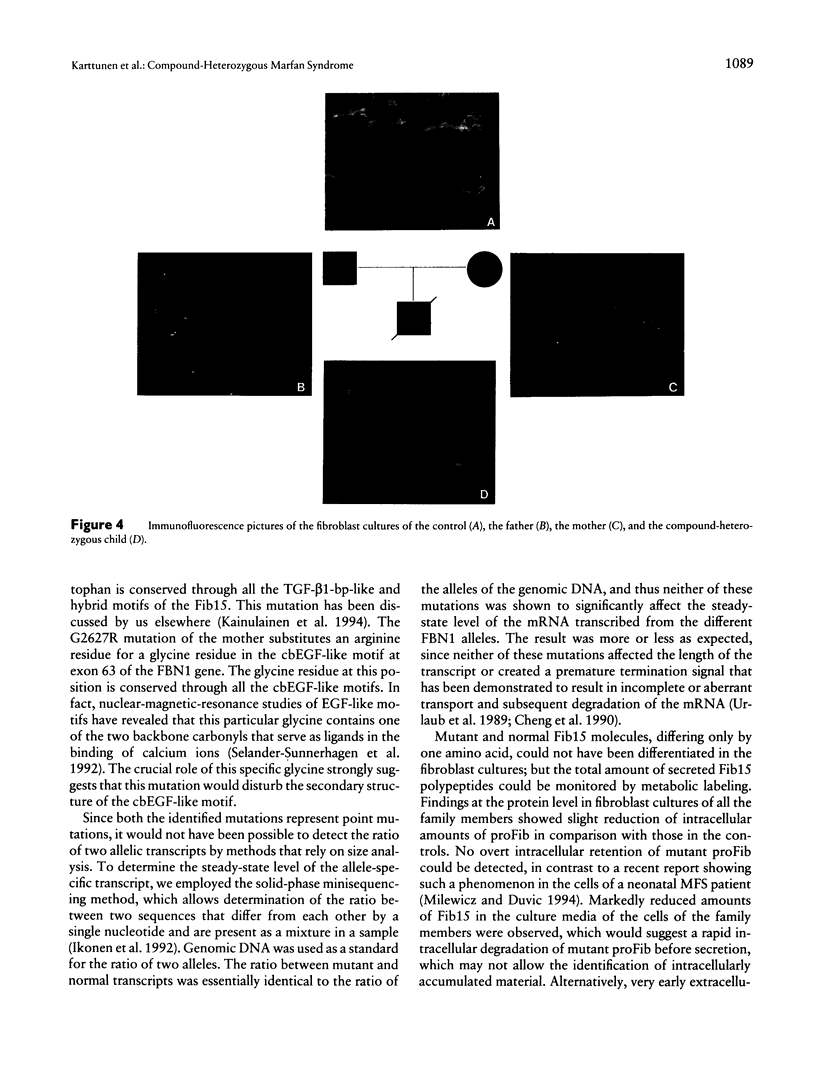


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandi M. L., Weber G., Svensson A., Falchetti A., Tonelli F., Castello R., Furlani L., Scappaticci S., Fraccaro M., Larsson C. Homozygotes for the autosomal dominant neoplasia syndrome (MEN1). Am J Hum Genet. 1993 Dec;53(6):1167–1172. [PMC free article] [PubMed] [Google Scholar]
- Buntinx I. M., Willems P. J., Spitaels S. E., Van Reempst P. J., De Paepe A. M., Dumon J. E. Neonatal Marfan syndrome with congenital arachnodactyly, flexion contractures, and severe cardiac valve insufficiency. J Med Genet. 1991 Apr;28(4):267–273. doi: 10.1136/jmg.28.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemke J., Nisani R., Feigl A., Garty R., Cooper M., Bårash Y., Duksin D. Homozygosity for autosomal dominant Marfan syndrome. J Med Genet. 1984 Jun;21(3):173–177. doi: 10.1136/jmg.21.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Fogel-Petrovic M., Maquat L. E. Translation to near the distal end of the penultimate exon is required for normal levels of spliced triosephosphate isomerase mRNA. Mol Cell Biol. 1990 Oct;10(10):5215–5225. doi: 10.1128/mcb.10.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Corson G. M., Chalberg S. C., Dietz H. C., Charbonneau N. L., Sakai L. Y. Fibrillin binds calcium and is coded by cDNAs that reveal a multidomain structure and alternatively spliced exons at the 5' end. Genomics. 1993 Aug;17(2):476–484. doi: 10.1006/geno.1993.1350. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Cutting G. R., Pyeritz R. E., Maslen C. L., Sakai L. Y., Corson G. M., Puffenberger E. G., Hamosh A., Nanthakumar E. J., Curristin S. M. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991 Jul 25;352(6333):337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., McIntosh I., Sakai L. Y., Corson G. M., Chalberg S. C., Pyeritz R. E., Francomano C. A. Four novel FBN1 mutations: significance for mutant transcript level and EGF-like domain calcium binding in the pathogenesis of Marfan syndrome. Genomics. 1993 Aug;17(2):468–475. doi: 10.1006/geno.1993.1349. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Saraiva J. M., Pyeritz R. E., Cutting G. R., Francomano C. A. Clustering of fibrillin (FBN1) missense mutations in Marfan syndrome patients at cysteine residues in EGF-like domains. Hum Mutat. 1992;1(5):366–374. doi: 10.1002/humu.1380010504. [DOI] [PubMed] [Google Scholar]
- Eikenboom J. C., Reitsma P. H., Peerlinck K. M., Briët E. Recessive inheritance of von Willebrand's disease type I. Lancet. 1993 Apr 17;341(8851):982–986. doi: 10.1016/0140-6736(93)91070-3. [DOI] [PubMed] [Google Scholar]
- Godfrey M., Vandemark N., Wang M., Velinov M., Wargowski D., Tsipouras P., Han J., Becker J., Robertson W., Droste S. Prenatal diagnosis and a donor splice site mutation in fibrillin in a family with Marfan syndrome. Am J Hum Genet. 1993 Aug;53(2):472–480. [PMC free article] [PubMed] [Google Scholar]
- Hewett D. R., Lynch J. R., Smith R., Sykes B. C. A novel fibrillin mutation in the Marfan syndrome which could disrupt calcium binding of the epidermal growth factor-like module. Hum Mol Genet. 1993 Apr;2(4):475–477. doi: 10.1093/hmg/2.4.475. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Manninen T., Peltonen L., Syvänen A. C. Quantitative determination of rare mRNA species by PCR and solid-phase minisequencing. PCR Methods Appl. 1992 May;1(4):234–240. doi: 10.1101/gr.1.4.234. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Karttunen L., Puhakka L., Sakai L., Peltonen L. Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nat Genet. 1994 Jan;6(1):64–69. doi: 10.1038/ng0194-64. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Sakai L. Y., Child A., Pope F. M., Puhakka L., Ryhänen L., Palotie A., Kaitila I., Peltonen L. Two mutations in Marfan syndrome resulting in truncated fibrillin polypeptides. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5917–5921. doi: 10.1073/pnas.89.13.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Milewicz D. M., Duvic M. Severe neonatal Marfan syndrome resulting from a de novo 3-bp insertion into the fibrillin gene on chromosome 15. Am J Hum Genet. 1994 Mar;54(3):447–453. [PMC free article] [PubMed] [Google Scholar]
- Milewicz D. M., Pyeritz R. E., Crawford E. S., Byers P. H. Marfan syndrome: defective synthesis, secretion, and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest. 1992 Jan;89(1):79–86. doi: 10.1172/JCI115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., D'Alessio M., Ramirez F., Lynch J. R., Sykes B., Pangilinan T., Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet. 1993 Jul;2(7):961–968. doi: 10.1093/hmg/2.7.961. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L., Kainulainen K., Krusius T., Mäkinen P., Schollin J., Gustavsson K. H., Peltonen L. Deficient expression of the gene coding for decorin in a lethal form of Marfan syndrome. J Biol Chem. 1990 Oct 15;265(29):17780–17785. [PubMed] [Google Scholar]
- Raghunath M., Superti-Furga A., Godfrey M., Steinmann B. Decreased extracellular deposition of fibrillin and decorin in neonatal Marfan syndrome fibroblasts. Hum Genet. 1993 Jan;90(5):511–515. doi: 10.1007/BF00217450. [DOI] [PubMed] [Google Scholar]
- Roa B. B., Garcia C. A., Pentao L., Killian J. M., Trask B. J., Suter U., Snipes G. J., Ortiz-Lopez R., Shooter E. M., Patel P. I. Evidence for a recessive PMP22 point mutation in Charcot-Marie-Tooth disease type 1A. Nat Genet. 1993 Oct;5(2):189–194. doi: 10.1038/ng1093-189. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Glanville R. W., Bächinger H. P. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991 Aug 5;266(22):14763–14770. [PubMed] [Google Scholar]
- Schollin J., Bjarke B., Gustavson K. H. Probable homozygotic form of the Marfan syndrome in a newborn child. Acta Paediatr Scand. 1988 May;77(3):452–456. doi: 10.1111/j.1651-2227.1988.tb10679.x. [DOI] [PubMed] [Google Scholar]
- Selander-Sunnerhagen M., Ullner M., Persson E., Teleman O., Stenflo J., Drakenberg T. How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X. J Biol Chem. 1992 Sep 25;267(27):19642–19649. doi: 10.2210/pdb1ccf/pdb. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Raghunath M., Willems P. J. Deficiencies of fibrillin and decorin in fibroblast cultures of a patient with neonatal Marfan syndrome. J Med Genet. 1992 Dec;29(12):875–878. doi: 10.1136/jmg.29.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Aalto-Setälä K., Harju L., Kontula K., Söderlund H. A primer-guided nucleotide incorporation assay in the genotyping of apolipoprotein E. Genomics. 1990 Dec;8(4):684–692. doi: 10.1016/0888-7543(90)90255-s. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Sajantila A., Lukka M. Identification of individuals by analysis of biallelic DNA markers, using PCR and solid-phase minisequencing. Am J Hum Genet. 1993 Jan;52(1):46–59. [PMC free article] [PubMed] [Google Scholar]
- Tynan K., Comeau K., Pearson M., Wilgenbus P., Levitt D., Gasner C., Berg M. A., Miller D. C., Francke U. Mutation screening of complete fibrillin-1 coding sequence: report of five new mutations, including two in 8-cysteine domains. Hum Mol Genet. 1993 Nov;2(11):1813–1821. doi: 10.1093/hmg/2.11.1813. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenplas S., Wiid I., Grobler-Rabie A., Brebner K., Ricketts M., Wållis G., Bester A., Boyd C., Måthew C. Blot hybridisation analysis of genomic DNA. J Med Genet. 1984 Jun;21(3):164–172. doi: 10.1136/jmg.21.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler N. S., Young A. B., Tanzi R. E., Travers H., Starosta-Rubinstein S., Penney J. B., Snodgrass S. R., Shoulson I., Gomez F., Ramos Arroyo M. A. Homozygotes for Huntington's disease. Nature. 1987 Mar 12;326(6109):194–197. doi: 10.1038/326194a0. [DOI] [PubMed] [Google Scholar]




