Abstract
An inhibitor of human liver glycogen phosphorylase a (HLGPa) has been identified and characterized in vitro and in vivo. This substance, [R-(R*,S*)]-5-chloro-N-[3-(dimethylamino)-2-hydroxy-3-oxo-1-(phenylmethyl)propyl]-1H-indole-2-carboxamide (CP-91149), inhibited HLGPa with an IC50 of 0.13 μM in the presence of 7.5 mM glucose. CP-91149 resembles caffeine, a known allosteric phosphorylase inhibitor, in that it is 5- to 10-fold less potent in the absence of glucose. Further analysis, however, suggests that CP-91149 and caffeine are kinetically distinct. Functionally, CP-91149 inhibited glucagon-stimulated glycogenolysis in isolated rat hepatocytes (P < 0.05 at 10–100 μM) and in primary human hepatocytes (2.1 μM IC50). In vivo, oral administration of CP-91149 to diabetic ob/ob mice at 25–50 mg/kg resulted in rapid (3 h) glucose lowering by 100–120 mg/dl (P < 0.001) without producing hypoglycemia. Further, CP-91149 treatment did not lower glucose levels in normoglycemic, nondiabetic mice. In ob/ob mice pretreated with 14C-glucose to label liver glycogen, CP-91149 administration reduced 14C-glycogen breakdown, confirming that glucose lowering resulted from inhibition of glycogenolysis in vivo. These findings support the use of CP-91149 in investigating glycogenolytic versus gluconeogenic flux in hepatic glucose production, and they demonstrate that glycogenolysis inhibitors may be useful in the treatment of type 2 diabetes.
Non-insulin-dependent diabetes mellitus (type 2 diabetes) is a prevalent disease in the Western world, afflicting ≈8 million diagnosed patients and a similar number of undiagnosed people in the United States alone (1). Although the cause of the commonly encountered form of type 2 diabetes has not yet been identified, it is well established that it is a polygenic disease characterized by multiple defects in insulin action in muscle, adipose, and liver, and defects in pancreatic insulin secretion (2). The relative importance of each of these in the etiology of type 2 diabetes is not clear. However, excessive hepatic glucose production (HGP) is a significant contributor to diabetic hyperglycemia. The liver is the major regulator of plasma glucose levels in the postabsorptive state, and in type 2 diabetics HGP is significantly elevated relative to nondiabetics (3, 4). In the postprandial state, where the liver has a proportionately smaller role in supplying glucose, the normal suppression of HGP is not observed in type 2 diabetics (4).
The liver produces glucose by two pathways, gluconeogenesis (de novo synthesis of glucose) and glycogenolysis (breakdown of glycogen by phosphorylase, EC 2.4.1.1). The relative contribution of each to net HGP in normal and diseased states has been difficult to quantitate (5–7), yet type 2 diabetics have been reported to display elevated gluconeogenic rates (3, 8). Attempts to modulate HGP with gluconeogenesis inhibitors have yielded mixed results. Agents that suppress gluconeogenesis in vitro or in diabetic rodents by reducing gluconeogenic substrate availability or fatty acid metabolism have generally not been clinically efficacious or safe in humans (9, 10). With the exception of metformin, an antidiabetic agent with multiple effects including gluconeogenesis inhibition, most inhibitors have failed to reduce HGP and plasma glucose levels in humans caused by hepatic autoregulation, a compensatory increase in hepatic glycogenolysis that maintains a high rate of HGP (9).
The alternative approach, the inhibition of glycogenolysis to reduce HGP, has not yet been tested. We hypothesized that glycogenolysis inhibition could improve glycemic control, based on patients with hepatic glycogen storage diseases, where episodic hypoglycemia is observed (11). Glucose production from the catalysis of glycogen to glucose-1-phosphate is rate-limited by phosphorylase a, a well-studied enzyme that is regulated by multiple covalent, substrate, and allosteric effectors (12). To date, two types of glycogen phosphorylase inhibitors have been reported: glucose analogs bearing multiple polar functionalities, which bind near the active site of the enzyme (13–18), and caffeine and other heteroaromatic analogs, which bind at the purine inhibitory site, also known as the I-site (19–22). Although some glucose and purine nucleoside phosphorylase inhibitors reportedly inhibit glycogenolysis in rodent cells and tissues (22–24), none of these are known to be orally active in vivo, possibly because of inadequate potency or poor pharmacokinetics, limiting their utility in determining the effect of glycogenolysis inhibition on HGP. Moreover, specific inhibitors of the human liver phosphorylase enzyme have not been described.
We chose to search for new compounds that inhibited the human liver glycogen phosphorylase a (HLGPa) enzyme to evaluate the basis of glycogenolysis inhibition for the treatment of type 2 diabetes. We hypothesized that inhibitors which bind at the I-site would be of most interest, because these compounds are reported to be more potent in the presence of high glucose concentrations (19–22). Inhibitory activity could then, in principle, be regulated by blood glucose levels and would decrease as normoglycemia is achieved. This characteristic should diminish the risk of hypoglycemia, a potential side effect of many antidiabetic agents. To find new inhibitors, we screened >300,000 compounds from our sample bank against recombinant HLGPa, and report here the discovery of an orally active inhibitor of HLGPa that lowers plasma glucose concentration in an animal model of type 2 diabetes.
MATERIALS AND METHODS
Expression and Purification of Recombinant HLGPa.
HLGP cDNA (25) was subcloned into plasmid pBlueBacIII (Invitrogen) and combined with BaculoGold Linear DNA (PharMingen) for baculovirus expression. HLGP was expressed in Sf9 cells as a mixture of phosphorylated (HLGPa) and unphosphorylated (HLGPb) forms and was purified by Cu2+ affinity chromatography (26); it was then reacted with phosphorylase kinase to convert all of the enzyme to the HLGPa form and subjected to a final step of anion exchange chromatography (D.E.D., unpublished data). The protein was >95% pure by SDS/PAGE and fully phosphorylated to HLGPa as judged by isoelectric focusing. The N terminus was correct as determined by protein sequencing on an Applied Biosystems model 470A sequencer.
Synthesis of CP-91149 ([R-(R*,S*)]-5-chloro-N-[3-(dimethylamino)-2-hydroxy-3-oxo-1-(phenylmethyl)propyl]-1H-indole-2-carboxamide).
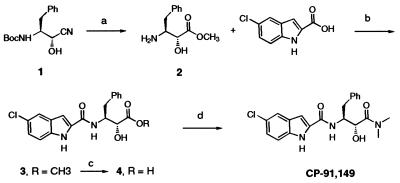
CP-91149 was synthesized as shown in Fig. 1. Cyanohydrin 1 (27) was treated with excess dry HCl in methanol, giving amino ester 2 (mp 106–107°C, ethyl acetate-hexanes) in high yield after aqueous extraction and recrystallization (28). Condensation of 2 with 5-chloro-1H-indole-2-carboxylic acid (1.0 equiv.) gave a 9:1 mixture of ester 3 and the diester resulting from N,O-bis-acylation of 2. Hydrolysis with aqueous NaOH in methanol and trituration of the crude extracted product with hot chloroform-ethyl acetate gave acid 4 (80% yield from 2). Coupling of 4 and dimethylamine HCl produced CP-91149 that was isolated by ethyl acetate extraction and purified by chromatography on silica gel (ethanol-dichloromethane). The colorless foam obtained was stirred in ether overnight, filtered, and dried, giving a colorless solid, mp 190–192°C (62% yield), homogeneous by 1H and 13C NMR, TLC, and RP-HPLC, crystalline by x-ray powder diffraction, and giving the expected LSIMS mass spectrum and carbon, hydrogen, and nitrogen (CHN) microanalysis. Complete experimental details and physical data (29) will be published separately.
Figure 1.
Synthetic route to CP-91149. a, HCl-MeOH, 60 h, 23°C, then NaHCO3/H2O, 77%; b, 1.0 equiv. 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide HCl, 1.5 equiv. 1-hydroxybenzotriazole, dichloromethane, 23°C, 18 h, 100% crude; c, 2.0 equiv. NaOH, MeOH, H2O, 25°C, 2 h, 80%; d, as for b, but 1.1 equiv. dimethylamine HCl and 1.1 equiv. triethylamine in dimethylformamide, 18 h, 25°C, 62%.
Phosphorylase Enzyme Assay.
HLGPa (85 ng) activity was measured in the direction of glycogen synthesis by the release of phosphate from glucose-1-phosphate (30) at 22°C in 100 μl of buffer containing 50 mM Hepes (pH 7.2), 100 mM KCl, 2.5 mM EGTA, 2.5 mM MgCl2, 0.5 mM glucose-1-phosphate, and 1 mg/ml glycogen. Phosphate was measured at 620 nm, 20 min after the addition of 150 μl of 1 M HCl containing 10 mg/ml ammonium molybdate and 0.38 mg/ml malachite green (31). Test compounds were added to the assay in 5 μl of 14% DMSO.
Glycogenolysis Inhibition in Rat Hepatocytes.
Isolated rat hepatocytes (32) were treated with CP-91149 or DMSO solvent (final concentration, 0.10%), followed by 60-min incubation with 0.3 nM glucagon (GGN). Assays were terminated by centrifugation, and cells were digested with 30% KOH followed by glycogen determination (33). Results are expressed as μmol glucose equivalent of glycogen/g weight.
Glycogenolysis Inhibition in Primary Human Hepatocytes.
Human hepatocytes (Clonetics, San Diego) were plated on collagen-coated 35-mm plates and maintained at 37°C and 95% O2/5% CO2 in Williams E Media (Clonetics) supplemented with 5 μg/ml insulin, 0.1 μM dexamethasone, 14 μM linoleic acid, 0.5 mg/ml BSA, and 2 mM glutamine (34). To radiolabel the glycogen pool for the assay, cells were incubated for 4 h with 0.2 μCi/ml [14C-U]glucose (NEC-042; 1 Ci = 37 GBq) plus 1 μM insulin. Cells were then washed with PBS, and basal and hormone-stimulated glycogen breakdown was examined in the absence or presence of 50 μM forskolin or 25 nM GGN, 10 mM glucose, plus CP-91149 as indicated. Assays were terminated 2 h later by washing with ice-cold PBS, solubilizing with 1 M NaOH, and quantitation of the 14C-glycogen (35). Results are expressed as dpm 14C-glycogen content per well.
Glucose Lowering in Vivo.
Five- to 6-week-old obese, diabetic male C57BL/6J-Lep(ob/ob) mice and their lean, nondiabetic C57BL/6J-?/+ littermates (The Jackson Laboratory) were housed under standard animal care practices with ad libitum access to food and water throughout the procedures. After 1-week acclimation, blood was collected from the retro-orbital sinus for plasma glucose determination (33), and mice were randomized to groups with similar mean ± SD. Mice were then dosed p.o. daily for 4 days with vehicle consisting of either (i) 0.25% (wt/vol) methyl cellulose in water or (ii) 0.1% Pluronic P105 Block Copolymer Surfactant (BASF, Parsippany, NJ) in 0.1% saline. On day 5, mice were treated p.o. with CP-91149 or vehicle, then bled 3 h later for plasma glucose determination. Statistical analysis of the CP-91149 effect was determined by unpaired t test with the vehicle-treated group. In some experiments, liver biopsies were obtained at 3 h postdosing for hepatic glycogen determination.
In Vivo Glycogenolysis.
The method of Liu et al. (36) for measuring in vivo glycogenolysis was modified for male C57BL/6J-Lepob mice by pretreatment with a liquid diet (5% glucose, 5% fructose, and 2% amino acids; ICN) for 48 h, followed by p.o. dosing of 0.17 μCi/g of 14C-glucose (NEC-042) to label the glycogen pool. After 3 h, mice were administered p.o. vehicle or CP-91149, then examined 3 h later for plasma glucose concentration and liver 14C-glycogen content (35). Statistical analysis of the CP-91149 effect was determined by unpaired t test with the vehicle-treated group.
RESULTS
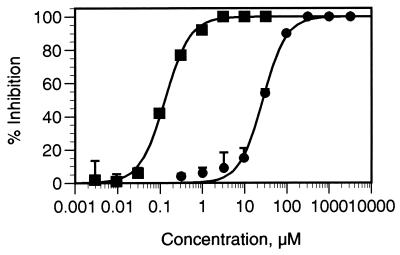
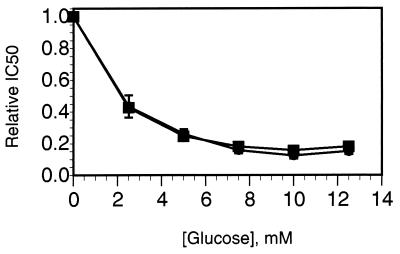
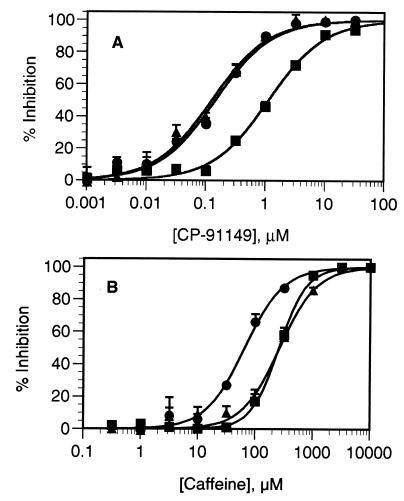
CP-91149 (Mr 399.86) was identified as a potent HLGPa inhibitor following screening of >300,000 compounds. Gram quantities of CP-91149 were prepared by a stereospecific synthetic route (Fig. 1), and the material obtained was determined to be pure (>98%, HPLC) and crystalline. Dose-response curves for HLGPa inhibition by CP-91149 and caffeine in the presence of a physiological glucose concentration (7.5 mM) are shown in Fig. 2. The IC50 for CP-91149 was calculated to be 0.13 μM, whereas the IC50 estimate for caffeine is 26 μM, 200-fold higher. The effect of varying glucose concentration on the IC50 values for both caffeine and CP-91149 is shown in Fig. 3. The data were normalized by dividing the IC50 obtained at varied glucose concentrations by the IC50 observed in the absence of glucose. At high glucose concentrations, the relative IC50 values for both CP-91149 and caffeine were decreased by 5- to 10-fold. Therefore, despite large differences in potency between CP-91149 and caffeine, glucose had the same relative effect on the IC50 estimates for both inhibitors. HLGPa inhibition by CP-91149 was also synergistic with caffeine and theophylline. To illustrate this, the IC50 values for CP-91149, caffeine and theophylline measured in the absence of glucose were found to be 1, 240, and 480 μM, respectively. Three buffers containing each of these compounds at their IC50 concentrations were prepared, and IC50 values were remeasured for CP-91149 and caffeine in these buffers. As shown in Fig. 4A, inclusion of caffeine or theophylline increased the potency of CP-91149 from 1 to 0.14 μM. Fig. 4B shows that the IC50 for caffeine was reduced in the presence of 1 μM CP-91149 from 240 to 68 μM; however, inclusion of 480 μM theophylline in the assay buffer had little effect on the IC50 value for caffeine. In additional experiments, HLGPa inhibition by theophylline was synergistic with CP-91149 but not with caffeine (data not shown).
Figure 2.
Inhibition of HLGPa by CP-91149 and caffeine. HLGPa activity was measured at varied concentrations of CP-91149 (▪) or caffeine (•). The data are plotted as percent inhibition vs. concentration of compound. The data (±SEM) are from a representative experiment performed in triplicate.
Figure 3.
The effect of glucose on the potency of CP-91149 (▪) and caffeine (•). IC50 values for HLGPa inhibition were determined at varied glucose concentrations, and then normalized by dividing the values by the IC50 value obtained in the absence of glucose. The normalized results are plotted as function of glucose concentration.
Figure 4.
Synergistic inhibition of HLGPa by CP-91149, caffeine and theophylline. (A) HLGPa activity was measured in the presence of varied concentrations of CP-91149 under control conditions (▪), or with 240 μM caffeine (•) or 480 μM theophylline (▴) in the assay buffer. (B) HLGPa activity was measured in the presence of varied caffeine concentration under control conditions (▪), or with 1.0 μM CP-91149 (•) or 490 μM theophylline (▴) in the assay buffer.
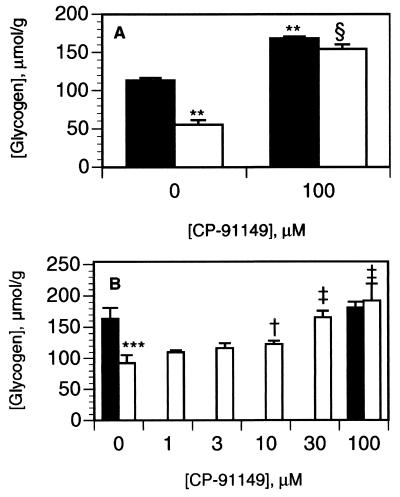
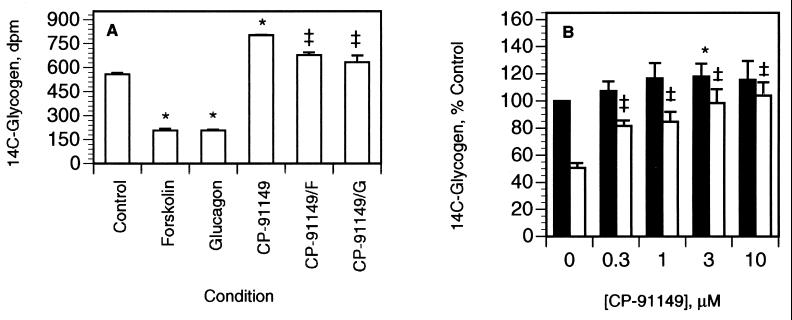
We next examined CP-91149 for phosphorylase inhibition in liver cells. In freshly isolated rat hepatocytes, the glycogenolytic hormone GGN reduced glycogen content by 50% (P < 0.01, Fig. 5A). Pretreatment of rat hepatocytes with 100 μM CP-91149 increased basal (no GGN) glycogen content by 48% (P < 0.01) and also markedly attenuated GGN-stimulated glycogenolysis (P < 0.01, Fig. 5A). CP-91149 inhibition of GGN-stimulated glycogenolysis was dose-dependent (Fig. 5B), with statistically significant effects observed at concentrations ≥10 μM (P < 0.05, Fig. 5B). A hormonally responsive primary human hepatocyte culture system was then developed for determining CP-91149’s effects on glycogenolysis by using 14C-glycogen prelabeled cells. Primary human hepatocytes displayed a 60% (P < 0.001) reduction in 14C-glycogen content in response to both GGN and forskolin, whereas coincubation with 30 μM CP-91149 inhibited the GGN and forskolin effect by >80% (P < 0.01) compared with untreated hepatocytes (Fig. 6A). GGN-stimulated glycogenolysis was inhibited (P < 0.05) by CP-91149 concentrations as low as 0.3 μM, with an estimated IC50 value of 2.1 μM (Fig. 6B). Treatment of the primary human hepatocytes with CP-91149 also increased basal 14C-glycogen content (44%, P < 0.001) compared with the control (Fig. 6A). Thus, CP-91149 suppressed glycogenolysis in both rat and human liver cells.
Figure 5.
Inhibition of glycogenolysis by CP-91149 in isolated rat hepatocytes. Isolated rat hepatocytes were preincubated with (A) 100 μM CP-91149 or DMSO or (B) 1–100 μM CP-91149 or DMSO for 5 min, then in the absence (filled bars) or presence (open bars) of 0.3 nM GGN for 60 min in buffer containing 7.5 mM glucose, followed by glycogen determination. Results (μmol/g) are the mean ± SD of triplicate determinations. ∗∗, P < 0.01; ∗∗∗, P < 0.001 between treatment (GGN, CP-91149) vs. control (no GGN); †, P < 0.05; ‡, P < 0.01; §, P < 0.001 between CP-91149 + GGN vs. control + GGN.
Figure 6.
Inhibition of glycogenolysis by CP-91149 in primary human hepatocytes. Primary human hepatocytes were incubated with [14C-U]glucose plus 1 μM insulin, then with 10 mM glucose and (A) 50 μM forskolin (F) or 25 nM GGN (G), plus 30 μM CP-91149 as indicated, or (B) in the absence (filled bars) or presence (open bars) of 25 nM GGN, plus CP-91149 as indicated for 2 h. (A) Results (dpm 14C-glycogen) are the mean ± SEM of triplicate determinations from a representative experiment. ∗, P < 0.001 between GGN, forskolin, or CP-91149 vs. untreated control; ‡, P < 0.01 between control + GGN or forskolin vs. CP-91149 + GGN or forskolin. (B) Results are expressed relative (%) to untreated control in the absence of GGN (100%) and are the mean ± SEM from 3 to 10 independent experiments, each performed in triplicate. ∗, P < 0.05 between CP-91149 vs. untreated control; ‡, P < 0.001 between CP-91149 + GGN vs. GGN-treated control.
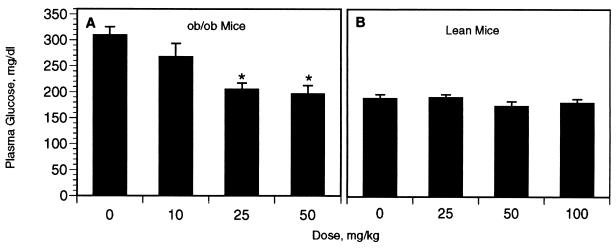
We next examined the effects of CP-91149 on glucose lowering and inhibition of glycogenolysis in vivo in diabetic ob/ob mice. Compared with vehicle-treated controls, dose-dependent decreases in plasma glucose concentrations (36–120 mg/dl) were observed 3 h after a single, oral dose of CP-91149 at 10, 25, and 50 mg/kg (Fig. 7A). Glucose lowering by CP-91149 was statistically significant (P < 0.01) at the 25 mg/kg and 50 mg/kg doses, and at 50 mg/kg, the glucose lowering reached normoglycemia (196 ± 16 mg/dl for the treated ob/ob mice vs. 189 ± 7 mg/dl for the nondiabetic controls, Fig. 7B). Hypoglycemia (glucose <60 mg/dl) was not noted in any of the mice dosed with CP-91149 (Fig. 7A). Administration of CP-91149 to normoglycemic, nondiabetic mice at 25–100 mg/kg did not effect glucose lowering (Fig. 7B).
Figure 7.
Dose-response of glucose lowering activity by CP-91149 in (A) obese, diabetic ob/ob mice, and (B) lean, nondiabetic control mice. Mice were treated with vehicle or CP-91149 as indicated, and plasma glucose concentration was determined 3 h later. Values (mg/dl) represent the mean ± SEM for 10–60 mice per group. ∗, Significant (P < 0.01) decrease by CP-91149 compared with the vehicle-treated group.
To determine whether glucose lowering by CP-91149 was associated with inhibition of glycogenolysis, liver glycogen content was compared between treated and control ob/ob mice. CP-91149 was associated with a 23% increase (P < 0.05) in net hepatic glycogen content over the 3-h treatment period during which glucose lowering (P < 0.001) was observed (Table 1). A sensitive method for measuring hepatic glycogenolysis in ob/ob mice, based on 14C-prelabeling of peripheral glucosyl residues of hepatic glycogen (36), was used to further assess glycogenolysis in vivo. Following prelabeling, the ob/ob mice were treated with either vehicle or 50 mg/kg CP-91149, and the residual 14C-liver glycogen content and plasma glucose concentration were measured 3 h later. As shown in Table 2, mice treated with CP-91149 had 3-fold higher (P < 0.01) 14C-liver glycogen content than the vehicle-treated control mice. In parallel, plasma glucose concentration was reduced by 150 mg/dl in the CP-91149 treated group (P < 0.001) compared with controls. Note that in Table 2, glycogenolysis was quantified by the differential loss of peripheral 14C-glucosyl residues from glycogen whereas in Table 1 the net (steady-state) glycogen content was measured. The differences between the two methods precludes a direct comparison of the quantitative effects; however, the results from the two studies are in agreement.
Table 1.
Effect of CP-91149 on net liver glycogen content and plasma glucose concentration in ob/ob mice
| Group | Net glycogen content, μmol/g | Plasma glucose concentration, mg/dl |
|---|---|---|
| Vehicle | 414 ± 30 | 303 ± 15 |
| CP-91149 | 510 ± 23* | 152 ± 7*** |
Liver biopsy samples (n=10) are from vehicle and 50 mg/kg CP-91149-treated ob/ob mice in Fig. 7. Results are the mean ± SEM. ∗, P < 0.05; ∗∗∗, P < 0.001 between CP-91149 and vehicle-treated groups.
Table 2.
Effect of CP-91149 on 14C-glycogen content and plasma glucose concentration in ob/ob mice
| Group | 14C-glycogen, dpm/mg | Plasma glucose concentration, mg/dl |
|---|---|---|
| Vehicle | 17.0 ± 2.6 | 235 ± 21 |
| CP-91149 | 51.6 ± 8.4** | 134 ± 7*** |
In vivo 14C-liver glycogenolysis was determined as described. Plasma glucose concentration and live 14C-glycogen content were determined 3 h after dosing. Values are the mean ± SEM for n = 9-10 mice from a representative experiment. ∗∗, P < 0.01; ∗∗∗, P < 0.001 between CP-91149 and vehicle-treated groups.
DISCUSSION
Our goal was to identify a HLGPa inhibitor that had cellular and in vivo activity to determine whether inhibition of glycogenolysis could be useful in the treatment of type 2 diabetes. Because inhibitors of HLGPa had not been previously described, we screened >300,000 compounds against a recombinant HLGPa enzyme, and identified CP-91149, an indole-containing small molecule (Fig. 1), that inhibited HLGPa with an IC50 of 0.13 μM in the presence of 7.5 mM glucose (Fig. 2). CP-91149 is structurally distinct from previously described glucose or I-site inhibitors (13–24), and is, in direct comparison with caffeine, 200-fold more potent. Despite the structural differences between CP-91149 and caffeine, we observed the same glucose synergy by these agents; HLGPa inhibition by both compounds increased 5- to 10-fold over a 0–10 mM glucose concentration range (Fig. 3). The effect of glucose on the potency of CP-91149 could be an important clinical feature of a glycogen phosphorylase inhibitor, because the reduction in inhibitor potency as glucose concentrations decrease in vivo should diminish the risk of hypoglycemia, a pervasive concern in type 2 diabetes therapy. This property could also help limit phosphorylase inhibition to the liver, because other tissues, such as skeletal muscle, have low intracellular glucose levels (37).
Several studies have shown that the glucose binding site and the I-site of glycogen phosphorylase can interact (19–22). Structural studies of muscle phosphorylase complexed with glucose/analogs and purine nucleoside inhibitors have shown that the glucose binding site is stabilized when the I-site is occupied (13, 38). Our data suggest that CP-91149 binds to a site that interacts with glucose in a manner similar to purine nucleoside effectors; however, preliminary biochemical experiments show that the binding characteristics of CP-91149 and purine nucleoside site inhibitors are not identical. CP-91149 inhibition of HLGPa was synergistic with both caffeine and theophylline (Fig. 4A), whereas caffeine inhibition was synergistic only with CP-91149, and not theophylline (Fig. 4B). These results indicate that CP-91149 is kinetically distinct from purine nucleoside ligands and further work is in progress to clarify this observation.
Based on the utility of rodent models in predicting human efficacy for antidiabetic drugs, and the high amino acid sequence identity (93.5%) of human and rat liver glycogen phosphorylase (39), we examined CP-91149 for glycogenolysis inhibition in both rat and human liver cells. CP-91149 inhibited GGN-stimulated glycogen breakdown in rat hepatocytes in a dose-dependent manner (Fig. 5B), and also suppressed GGN-stimulated glycogenolysis in primary human hepatocytes, with an IC50 of ≈2.1 μM (Fig. 6B). The functional activity displayed in hepatocytes indicated that CP-91149’s inhibitory effects could potentially translate in vivo, so CP-91149 was administered to diabetic ob/ob mice based on their similar phenotype to human type 2 diabetics (40). We observed that a single, oral dose of CP-91149 at 50 mg/kg reduced plasma glucose concentrations in ob/ob mice to near-normal levels 3 h after dosing; significant glucose lowering was also obtained at the 25 mg/kg dose level (Fig. 7A). In no case was hypoglycemia observed. In nondiabetic, normoglycemic mice, administration of CP-91149 at doses up to 100 mg/kg, 4-fold higher than the lowest efficacious dose in diabetic mice, did not result in glucose lowering (Fig. 7B). The reduced potency of CP-91149 in the normoglycemic mice paralleled the compound’s loss of inhibitory potency for HLGPa at reduced glucose concentrations in vitro (Fig. 3).
The glucose-lowering elicited by CP-91149 treatment was accompanied by an inhibition of hepatic glycogen breakdown in the diabetic ob/ob mice (Tables 1 and 2). This effect is consistent with inhibition of hepatic glycogen phosphorylase in vivo, but cannot exclude one or more additional mechanisms. However, we have found that CP-91149 is highly specific for HLGPa inhibition, based on broad in vitro testing for activity against other potential targets, including enzymes that regulate glucose metabolism. CP-91149 (up to 10–100 μM) did not inhibit phosphoenolpyruvate carboxykinase, glucose-6-phosphatase/translocase, phosphoglucomutase, glucose-6-phosphate dehydrogenase, or fructose-1,6-bisphosphatase activities (results not shown). Further, CP-91149 did not show activity in at least 50 other pharmacological target screens, including at least 13 additional diabetes-related targets not mentioned above. In addition, the 3(S),2(S) diasteromer of CP-91149, a closely related analog with sharply reduced HLGPa inhibition, did not show glucose lowering activity in vivo at 50 mg/kg. We have also ruled out other nonhepatic mechanisms of glucose lowering with active CP-91149 analogs by demonstrating that these compounds do not stimulate glucose uptake in L6 myocytes, nor stimulate insulin secretion in rat islets at concentrations up to 30 μM (E. M. Gibbs and J. C. Parker, personal communication). This result is in contrast to CP-91149’s marked inhibition of human hepatocyte glycogenolysis at concentrations as low as 0.3 μM. Taken together, our results suggest that hepatic glycogenolysis is quantitatively important to HGP and glycemic control in the diabetic ob/ob mouse, and that phosphorylase inhibition can restore euglycemia in this hyperglycemic model. Longer term studies are in progress to determine if additional metabolic benefits are derived from glycogen phosphorylase inhibition, and whether a phenomenon of hepatic autoregulation (9) will be induced.
The results from studies of the relative contributions of glycogenolysis vs. gluconeogenesis to HGP have been contradictory (6). Even recent work with new methodologies does not give a clear answer. NMR (5, 41) or deuterium enrichment (8) studies support a dominant role for gluconeogenesis, whereas mass isotopomer distribution analysis predicts a larger role for glycogenolysis (42). Our study demonstrates that the pharmacological inhibition of hepatic glycogenolysis results in significant glucose lowering in diabetic rodents, suggesting that glycogenolysis is quantitatively important to HGP. This issue is further complicated, however, by recent studies reporting that a substantial portion of de novo glucose (e.g., up to 78% by mass isotopomer) appears to cycle through the glycogen pool prior to efflux from human liver (43). NMR data also corroborate that significant hepatic glucose-glycogen cycling occurs in vivo (44, 45). These data support the conjecture that glycogenolysis inhibition could also reduce net gluconeogenic flux, and thereby contribute to the overall efficacy in reducing HGP in vivo. In preliminary experiments utilizing fasted rat hepatocytes, we observed that CP-91149 (30 μM) reduced 14C-lactate conversion to 14C-glucose by 50%, simultaneous with 3-fold increased 14C-lactate incorporation into 14C-glycogen (results not shown), despite the compound’s lack of direct inhibition of several key gluconeogenic enzymes. These results indicate that CP-91149 may indirectly inhibit gluconeogenesis as a result of primary inhibition of glycogenolysis, and additional studies are underway to address this point.
In conclusion, an inhibitor of human liver glycogen phosphorylase is reported that functionally inhibits GGN-stimulated glycogenolysis in rat hepatocytes, and in human hepatocytes, the potential therapeutic target. Our data show that an orally active pharmacological agent can both inhibit hepatic glycogenolysis and lower plasma glucose levels in diabetic rodents. The results indicate that hepatic glycogenolysis has a major role in the regulation of plasma glucose levels in diabetic mice, and suggest that glycogen phosphorylase inhibitors may be useful in the treatment of type 2 diabetes.
Acknowledgments
We thank Robert J. Fletterick for advice and provision the HLGP cDNA. The technical contribution of Eric S. Eberhardt in the first synthesis of CP-91149 was essential. We also thank Mark Ammirati, Joseph Russo, Pia Vestergaard, and Carolyn Levy for technical assistance, and William G. Stirtan, Virgina L. Rath, Younggil Kwon, E. Michael Gibbs, and Gregory D. Berger for critical reading and suggestions during the course of this work.
ABBREVIATIONS
- HGP
hepatic glucose production
- HLGPa
human liver glycogen phosphorylase a
- CP-91149
[R-(R*,S*)]-5-chloro-N-[3-(dimethylamino)-2-hydroxy-3-oxo-1-(phenylmethyl)propyl]-1H-indole-2-carboxamide
- GGN
glucagon
References
- 1.Kenny S J, Aubert R E, Geiss L S. In: Diabetes in America. Harris M, editor. Bethesda, MD: National Institutes of Health; 1995. , NIH publication 95-1468, 2nd Ed., pp. 47–67. [Google Scholar]
- 2.DeFronzo R A, Bonadonna R C, Ferrannini E. Diabetes Care. 1992;15:318–369. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Consoli A. Diabetes Care. 1992;15:430–441. doi: 10.2337/diacare.15.3.430. [DOI] [PubMed] [Google Scholar]
- 4.Gerich, J. E. (1992) Horm. Metab. Res 26 (Suppl.), 18–21. [PubMed]
- 5.Rothman D E, Magnusson I, Katz L D, Shulman R G, Shulman G I. Science. 1991;254:573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 6.Barrett E J, Liu Z. Balliere’s Clin Endocrinol Metab. 1993;7:875–901. doi: 10.1016/s0950-351x(05)80238-1. [DOI] [PubMed] [Google Scholar]
- 7.Pimenta W, Nurjhan N, Jansson P-A, Stumvall M, Gerich J, Korytkowski M. Diabetologia. 1994;37:697–702. doi: 10.1007/BF00417694. [DOI] [PubMed] [Google Scholar]
- 8.Landau B R, Wahren J, Chadramouli V, Schumann W C, Ekberg K, Kalhan S C. J Clin Invest. 1996;98:378–385. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yki-Javinen H. Diabetes Nutr Metab. 1994;7:109–119. [Google Scholar]
- 10.Bressler R, Johnson D. Diabetes Care. 1992;15:792–805. doi: 10.2337/diacare.15.6.792. [DOI] [PubMed] [Google Scholar]
- 11.Hers H G. Rev Int Hepatol. 1959;9:35–55. [PubMed] [Google Scholar]
- 12.Newgard C B, Hwang P K, Fletterick R J. Crit Rev Biochem Mol Biol. 1989;24:69–99. doi: 10.3109/10409238909082552. [DOI] [PubMed] [Google Scholar]
- 13.Martin J L, Veluraja K, Ross K, Johnson L N, Fleet G W J, Ramsden N G, Bruce I, Orchard M G, Oikonomakos N G, Papageorgiou N G, Leonidas D D, Tsitoura H S. Biochemistry. 1991;30:10101–10116. doi: 10.1021/bi00106a006. [DOI] [PubMed] [Google Scholar]
- 14.Watson K A, Mitchell E P, Johnson L N, Son J C, Bichard C J F, Orchard M G, Fleet G W J, Oikonomakos N G, Leonidas D D, Kontou M, Papageorgioui A. Biochemistry. 1994;33:5745–5758. doi: 10.1021/bi00185a011. [DOI] [PubMed] [Google Scholar]
- 15.Bichard C J F, Mitchell E P, Wormald M R, Watson K A, Johnson L N, Zographos S E, Koutra D D, Oikonomakos N G, Fleet G W J. Tetrahedron Lett. 1995;36:2145–2148. [Google Scholar]
- 16.Krulle T M, Watson K A, Gregoriou M, Johnson L N, Crook S, Watkin D J, Griffiths R C, Nash R J, Titanou K E, Zographos S E, Oikonomakos N G, Fleet G W J. Tetrahedron Lett. 1995;36:8291–8294. [Google Scholar]
- 17.Lundgren, K. & Kirk, O. (1995) Patent No. WO 95/24391-A1.
- 18.Lundgren, K., Jakobsen, P., Kristiansen, M., Norskov, L. L. & Naerum, L. (1997) Patent No. WO 97/09040-A1.
- 19.Kasvinsky P J, Madsen N B, Sygusch J, Fletterick R J. J Biol Chem. 1978;253:3343–3351. [PubMed] [Google Scholar]
- 20.Kasvinsky P J, Shechosky S, Fletterick R J. J Biol Chem. 1978;253:9102–9106. [PubMed] [Google Scholar]
- 21.Ercan-Fang N, Nuttall F Q. J Pharmacol Exp Ther. 1997;280:1312–1318. [PubMed] [Google Scholar]
- 22.Kasvinsky P J, Fletterick R J, Madsen N B. Can J Biochem. 1981;59:387–395. doi: 10.1139/o81-054. [DOI] [PubMed] [Google Scholar]
- 23.Board M, Hadwen M, Johnson L N. Eur J Biochem. 1995;228:753–761. doi: 10.1111/j.1432-1033.1995.0753m.x. [DOI] [PubMed] [Google Scholar]
- 24.Board M, Bollen M, Stalmans W, Kim Y, Fleet G W J, Johnson L N. Biochem J. 1995;311:845–852. doi: 10.1042/bj3110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coats W S, Browner M F, Fletterick R F, Newgard C B. J Biol Chem. 1991;266:16113–16119. [PubMed] [Google Scholar]
- 26.Luong C B, Browner M F, Fletterick R J. J Chromatogr. 1992;584:77–84. doi: 10.1016/0378-4347(92)80011-e. [DOI] [PubMed] [Google Scholar]
- 27.Parris K D, Hoover D J, Damon D B, Davies D R. Biochemistry. 1992;31:8125–8141. doi: 10.1021/bi00150a004. [DOI] [PubMed] [Google Scholar]
- 28.Hoover, D. J. (1993) Patent Application WO 9325574 A1 931223.
- 29.Hulin, B., Hoover, D. J., Treadway, J. L. & Martin, W. H. (1996) Patent Application WO 9639385 A1 961212.
- 30.Engers H D, Shechosky S, Madsen N B. Can J Biochem. 1970;48:746–754. doi: 10.1139/o70-117. [DOI] [PubMed] [Google Scholar]
- 31.Lanzetta P A, Alvarez L J, Reinach P S, Candia O A. Anal Biochem. 1979;100:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- 32.Blackmore P F, McPherson R K, Stevenson R W. Metabolism. 1993;42:1583–1587. doi: 10.1016/0026-0495(93)90154-g. [DOI] [PubMed] [Google Scholar]
- 33.Treadway J L, Hargrove D M, Nardone N A, McPherson R K, Russo J F, Milici A J, Stukenbok H A, Gibbs E M, Stevenson R W, Pessin J E. J Biol Chem. 1994;269:29956–29961. [PubMed] [Google Scholar]
- 34.Dorko K, Freeswick P D, Bartoli F, Cicalese L, Bardsley B A, Tzakis A, Nussler A K. Cell Transplant. 1994;3:387–395. doi: 10.1177/096368979400300505. [DOI] [PubMed] [Google Scholar]
- 35.Garetto L P, Richter E A, Goodman M N, Ruderman N B. Am J Physiol. 1984;246:E471–E475. doi: 10.1152/ajpendo.1984.246.6.E471. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Gardener L B, Barrett E J. Metabolism. 1993;42:1546–1551. doi: 10.1016/0026-0495(93)90149-i. [DOI] [PubMed] [Google Scholar]
- 37.Goodman M N, Dluz S M, McElaney M A, Belur E, Ruderman N B. Am J Physiol. 1983;244:E93–E100. doi: 10.1152/ajpendo.1983.244.1.E93. [DOI] [PubMed] [Google Scholar]
- 38.Sprang S, Fletterick R, Stem M, Yang D, Madsen N, Sturtevant J. Biochemistry. 1982;21:2036–2048. doi: 10.1021/bi00538a010. [DOI] [PubMed] [Google Scholar]
- 39.Schiebel K, Pekel E, Mayer D. Biochim Biophys Acta. 1992;1130:349–351. doi: 10.1016/0167-4781(92)90453-7. [DOI] [PubMed] [Google Scholar]
- 40.Herberg L, Coleman D L. Metabolism. 1977;26:59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- 41.Magnusson I, Rothman D L, Katz L, Shulman R G, Shulman G I. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tayek J A, Katz J. Am J Physiol. 1996;270:E709–E717. doi: 10.1152/ajpendo.1996.270.4.E709. [DOI] [PubMed] [Google Scholar]
- 43.Hellerstein M K, Neese R A, Linfoot P, Christiansen M, Turner S, Letscher A. J Clin Invest. 1997;100:1305–1319. doi: 10.1172/JCI119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David M, Petit W A, Laughlin M R, Schulman R G, King J E, Barrett E J. J Clin Invest. 1990;86:612–617. doi: 10.1172/JCI114752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roden M, Perseghin G, Petersen K F, Hwang J-H, Cline G W, Gerow K, Rothman D L, Shulman G I. J Clin Invest. 1996;97:642–648. doi: 10.1172/JCI118460. [DOI] [PMC free article] [PubMed] [Google Scholar]