Abstract
The enzyme cyclooxygenase (COX)-1 is constitutive whereas COX-2 is regulated in virtually all tissues. To assess whether this dogma holds true in the pancreatic islet, we examined basal and interleukin (IL)-1-regulated expression of COX-2 in HIT-T15 cells, Syrian hamster and human islets, and other Syrian hamster tissues. We found that COX-2, and not COX-1, gene expression is dominant in pancreatic islet tissue under both basal and IL-1-stimulated conditions. Control tissues (liver, spleen, and kidney) showed the expected predominance of COX-1 gene expression. Basal and IL-1-stimulated prostaglandin E2 synthesis were blocked by a specific COX-2 inhibitor. IL-1 stimulation had a biphasic effect on COX-2 mRNA levels with an initial mild increase at 2–4 hr followed by a more dramatic decrease below basal level by 24 hr. The IL-1-induced increase in COX-2 mRNA levels was accompanied by a parallel increase in NF-κB binding to COX-2 promoter elements. The subsequent decrease in COX-2 mRNA levels was accompanied by a parallel decrease in NF-IL-6 binding activity and COX-2 promoter activity. Specific mutation of the NF-IL-6 binding motif within the COX-2 promoter reduced basal promoter activity by 50% whereas mutation of the NF-κB motif had no effect. These studies provide documentation of NF-IL-6 in the pancreatic islet and that COX-2, rather than COX-1, is dominantly expressed. They suggest coordinate regulation by IL-1 of COX-2 mRNA, NF-κB, and NF-IL-6 and raise the issue of whether intrinsically high levels of COX-2 gene expression predisposes the normal islet for microenvironmentally induced overproduction of islet prostaglandin E2.
In virtually all tissues cyclooxygenase (COX)-1 is the basal or constitutive form of the enzyme whereas COX-2 is the regulated or inducible form expressed during stressful processes, such as inflammation, or after exposure to 10% fetal bovine serum (FBS) (presumably because it contains cytokines). Early work by Rosen et al. (1) and Fu et al (2) suggested that two genes may exist that are responsible for synthesis of COX-1 and COX-2, respectively. In later experiments using synovial tissue from patients with rheumatoid arthritis, Crofford et al. (3) showed that de novo synthesis of COX-2 is enhanced by interleukin (IL)-1 and that this enhancement is markedly suppressed by dexamethasone. These investigators further demonstrated that COX-2 mRNA was greatly increased by IL-1 and suppressed by dexamethasone. Other work has demonstrated differential sensitivity of COX-1 and COX-2 activities to inhibition by nonsteroidal anti-inflammatory drugs (4, 5).
The sequence of the COX-2 promoter is known, and multiple putative cis-acting regulatory elements have been identified (6). Among these elements are consensus elements for NF-κB and nuclear factor (NF)-IL-6. These two factors are important mediators of IL-1 action on gene transcription in cellular systems (7, 8). Inflammatory cytokines (i.e., IL-1, tumor necrosis factor α, interferon γ, and IL-6) are likely to play important autacrine or paracrine roles during the onset of diabetes mellitus at the time of the autoimmune response (reviewed in refs. 9 and 10). IL-1 is known to influence a wide array of metabolic processes within the beta cell, including insulin content and release (11), mitochondrial oxidative activity (12), calcium uptake (13), phosphoinositide metabolism (14), total protein synthesis, and ATP/ADP ratio (15). In many of these cases, the effect of IL-1 on the beta cell is bimodal with an initial stimulatory phase, lasting for a matter of hours, followed by progressive inhibition. The potential of IL-1 to regulate COX gene expression is also highly relevant to pancreatic islet function because arachidonic acid metabolites, principally prostaglandin E2 (PGE2), modulate beta cell function (16, 17).
We designed the experiments reported herein to assess in the pancreatic islet the proposition that COX-1 gene expression is constitutive and that COX-2 gene expression is inducible by IL-1 and suppressible by dexamethasone. Contrary to prevailing theory, we found that COX-2, and not COX-1, mRNA and protein activity are dominantly expressed in HIT-T15 cells, hamster islets, and human islets in the basal state. IL-1 induction of COX-2 gene expression was bimodal, inhibited by dexamethasone, and associated with alterations in NF-IL-6 and NF-κB activities. We suggest that this propensity for increased COX-2 gene expression in the basal state may render the islet intrinsically more likely to respond to microenvironmental increases in IL-1 by increasing PGE2 production (18–21).
MATERIALS AND METHODS
Cell Culture and Pancreatic Islet and Hepatocyte Preparation.
Stock cultures of HIT-T15 cells (passages 72–79) were routinely grown in 5% CO2/95% O2 humidified air at 37°C, maintained in RPMI 1640 medium supplemented with 10% FBS, passed once weekly after detachment by using trypsin-EDTA, and fed every 48 hr by changing medium as previously described (22). Before experiments, cells were maintained in RPMI medium containing 0.2% FBS for 24 hr. Islets and hepatocytes were isolated by using modifications of previously described methods (23, 24) involving pancreatic distention with collagenase and purification on a discontinuous Ficoll gradient. Islets and acinar debris from the 20.5%–11% interface were handpicked by using a fine pipette. Nonislet tissues (spleen, liver, and kidney) were freshly dissected, pulverized in liquid nitrogen, and homogenized in guanidinium thiocyanate solution followed by RNA isolation.
Northern Analysis of Poly(A) mRNA from HIT-T15 Cells.
Cell monolayers were washed twice with PBS, then lysed in 4 M guanidinium thiocyanate solution followed by RNA extraction. Total RNA was extracted from cells by guanidinium thiocyanate (25). Poly(A) mRNA was isolated from total RNA by using the PolyATtract mRNA Isolation System (Promega), separated in a 1.5% agarose-formaldehyde gel, and transferred to a nylon membrane by electroblotting. After UV crosslinking the membrane was prehybridized overnight and then hybridized with random primer 32P-labeled mouse COX-1 or COX-2 cDNA probes. cDNA probes were gifts from David L. DeWitt, Michigan State University, Lansing, MI (mouse COX-1 and COX-2 probes) and Timothy Hla, American Red Cross, Rockville, MD (human COX-2 probe).
Reverse Transcriptase–PCR (RT-PCR) Using Syrian Hamster Tissue and Human Islets.
Total RNA (2 μg) was reverse-transcribed to cDNA by incubation at 37°C for 1 hr in 50 μl of reverse transcription buffer (50 mM Tris⋅HCl, pH 8.3/75 mM KCl/3 mM MgCl2/20 μM DTT) with 0.5 μg of oligo(dT)12–18 primer, 33 units of RNasin (Promega), 400 units of M-murine leukemia virus reverse transcriptase, and 4.0 mM dNTPs. Single-stranded cDNA synthesized from the total RNA was used for PCR amplification with COX-1, COX-2, and transcription factor II D (TFIID)-specific primers. Oligonucleotide primers for HIT cell and hamster tissue COX-1 and COX-2 cDNAs were synthesized based on the consensus sequence of mouse. For COX-1 cDNA, sense and antisense primers were 5′-CTCACAGTGCGGTCCAAC-3′ and 5′-CCAGCACCTGGTACTTAAG-3′, giving rise to a 424-bp PCR product. For COX-2 cDNA, primers were 5′-TTCAAAAGAAGTGCTGGAAAAGGT-3′ and 5′-GATCATCTCTACCTGAGTGTCTTT-3′, giving rise to a 304-bp PCR product. The primers for human COX-2 cDNA were 5′-TTCAAATGAGATTGTGGGAAAAT-3′ and 5′-AGATCATCTCTGCCTGAGTATCTT-3′, giving rise to a 305-bp PCR product. As a control, TFIID primers were 5′-CCACAGCCTATTCAGAAC-3′ and 5′-GCTCCTGTGCACACCAT-3′, giving rise to 487-bp and 556-bp PCR product for human and hamster, respectively. The amplification reactions were carried out in a Perkin–Elmer GeneAmp PCR System 2400 for 30 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1.5 min. The RT-PCR products were detected by 6% nondenaturing PAGE and visualized by silver staining (26). RT-PCR products for COX-1 and COX-2 were found on sequencing by dye terminator cycle sequencing kit (Perkin–Elmer) to be 90 and 92% identical, respectively, to mouse COX-1 and COX-2 cDNAs.
Oligonucleotides.
The following oligonucleotides were synthesized by GIBCO/BRL: human NF-IL-6 sense, 5′-CCCCACCGGCTTACGCAATTTTTTTAAGG-3′; human NF-IL-6 antisense, 5′-CCTTAAAAAAATTGCGTAAGCCGGTGGGG-3′; human NF-κB sense, 5′-GCGGGAGAGGGGATTCCCTGCGGCCCCG-3′; human NF-κB antisense, 5′-CGGGGCCGCAGGGAATCCCCTCTCCCGC-3′; rat NF-IL-6 sense, 5′-TGGGTATTATGCAATTGGAAG-3′; rat NF-IL-6 antisense, 5′-CTTCCAATTGCATAATACCCA-3′; rat NF-IL-6 mutant sense, 5′-CCCCTATGGGTATACGATTGGAAGCGGAGA-3′; rat NF-IL-6 mutant antisense, 5′-TCTCCGCTTCCAATCGTATACTACCCATAGGGG-3′; rat NF-κB mutant sense, 5′-GGGAGAGGCAAGGGCCCTCCCTTAGTTAGGATC-3′; and rat NF-κB mutant antisense, 5′-GATCCTAACTAAGGGAGGGCCCTTGCCTCTCCC-3′.
For electrophoretic mobility shift assay (EMSA), double-stranded probes were made by annealing complementary oligonucleotides followed by 32P end-labeling by T4 polynucleotide kinase. Unincorporated nucleotides were removed by using a Bio-Rad 6 Spin column. For studies of promoter activity, successful mutation of the NF-κB binding site was confirmed by the insertion of a novel ApaI site (designed into the mutant NF-κB annealing primer) within the COX-2 promoter. Similarly, successful mutation of the NF-IL-6 site resulted in the novel insertion of an ACC I site detectable by restriction digest analysis.
EMSA.
Ten micrograms of nuclear extract protein were incubated for 30 min at room temperature with 30,000 cpm of labeled oligodeooxynucleotide probe in the presence or absence of unlabeled competitor probe or mutant probe as indicated under binding conditions (5% glycerol/1 mM EDTA/50 mM NaCl/1 mM Tris⋅HCl, pH 7.5/1 mM DTT). Samples were separated on a 4.5% polyacrylamide gel running at 200 V for 2 hr. Specific antisera recognizing C/EBP-α, C/EBP-β (NF-IL-6), NF-κB p65, and NF-κB p50 were purchased from Santa Cruz Biotechnology.
Transfection Assay.
Plasmid DNA was purified from a DH5-alpha Escherichia coli host strain (GIBCO/BRL) by using the Qiagen Maxi-Prep Isolation system. Integrity of the plasmid DNA was confirmed by restriction digest mapping based on the published sequence of the construct (27). Purified plasmid DNA (1.0 μg) was transfected by using lipofectin reagent (GIBCO/BRL).
Chloramphenicol Acetyltransferase (CAT) Activity Assay.
Once harvested, cells were resuspended in 100 μl of 250 mM Tris⋅HCl, pH 7.5 and lysed by three repetitive freeze-thaw cycles with intermittent vortexing. After precipitation and assay of protein concentration using BCA reagent, supernatant was stored at −70°C. One hundred microliters of reaction mixture (250 mM Tris⋅HCl/10 mM acetyl-CoA/14C-chloramphenicol) was added to 50 μl of sample containing 25–50 μg of protein. Reaction was allowed to proceed at 37°C for 4 hr. After ethyl acetate extraction, chromatography was carried out for 1 hr in chloroform/methanol (76:5), and the plate was exposed to film overnight at −70°C. Quantitation of CAT activity was through scintillation analysis of acetylated species. The COX-2/CAT reporter gene construct was the gift of JoAnne Richards (Baylor College of Medicine, Houston, TX). Generation of the construct (27) involved the subcloning of the fragment −2,698/+32 of the rat COX-2 gene upstream of the CAT reporter gene in the pCAT Basic Vector (Promega). Selective mutation of the NF-κB and NF-IL-6 sites within the COX-2/CAT reporter gene construct was accomplished by using the QuickChange Site-Directed Mutagenesis Kit (Stratagene).
Western Analysis of Human Islets.
Cells were washed twice with 1.5 ml of Tris-buffered saline (135 mM NaCl/3 mM KCl/12 mM Tris⋅HCL, ph 7.4) and then lysed with 1 ml of lysis buffer (20 mM Tris⋅HCL, pH 7.5/16 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/1 mM EDTA/1 mM benzamidine/10 μg/ml soy trypsin inhibitor/1 μg/ml leupeptin). Cell debris were removed by centrifugation. The supernatant was transferred to a new tube and assayed for protein by the BCA method (Pierce, Rockford, IL). Proteins were separated by SDS/PAGE with an 8% acrylamide gel. Gels were 16 cm long and 0.75 mm thick and run at 25 mA per gel for 5 hr. Proteins were transferred to Immobilon P membranes (Millipore) by semidry transfer and then subjected to Western analysis. For immunoblotting, membranes were blocked as previously described (28). Mixed monoclonal COX-1 antiserum was used at a 1:250 dilution; polyclonal COX-2 antiserum was used at a 1:7,500 dilution (29). Immunoreactivity was detected by applying a horseradish peroxidase-conjugated second antibody and treating with a chemiluminescence substrate (Pierce). The treated membranes were exposed to X-Omat AR x-ray film for varying amounts of time from 30 sec to 5 min.
PGE2 Enzymeimmunoassay.
Cells were incubated in RPMI 1640 medium without phenol red (GIBCO) containing 0.2% FBS in the absence or in the presence of the specific COX-2 inhibitor NS-398 (0.01 mM; Biomol, Plymouth Meeting, PA) for the designated times. Accumulated PGE2 was measured in the culture media by enzymeimmunoassay (Amersham).
RESULTS
COX-1 vs. COX-2 Gene Expression.
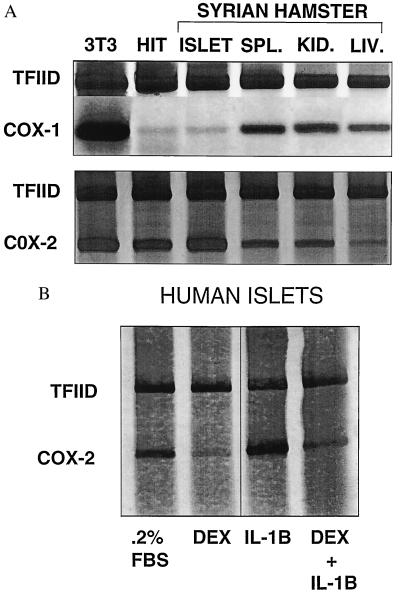
When using poly(A) mRNA preparations analyzed by Northern blot, COX-1 mRNA levels in HIT cells cultured in 0%, 0.2%, or 10% FBS were virtually nonmeasurable whereas COX-1 mRNA levels in 3T3 cells cultured in 0.2% FBS were readily detectable (Fig. 1). However, COX-2 mRNA levels in HIT cells cultured in 0% or 0.2% FBS were readily detectable and increased in HIT cells cultured in 10% FBS (Fig. 1). Exposure of hepatocytes for 1 hr to collagenase and Ficoll (materials used to isolate islets) did not cause COX-2 gene expression (data not shown). When using RT-PCR, COX-1 mRNA was barely detectable in HIT cells and Syrian hamster islets whereas it was readily detectable in 3T3 cells and Syrian hamster spleen, kidney, and liver in the presence of 10% FBS. On the other hand, RT-PCR readily demonstrated COX-2 mRNA in HIT cells and hamster islets and detected lesser amounts in Syrian hamster spleen, kidney, and liver (Fig. 2A). When using Northern analysis of total RNA (because of limited amounts of human tissue), COX-1 mRNA was nondetectable, whereas COX-2 mRNA was detectable, in human islets cultured in 0.2% FBS without and with IL-1 stimulation for 4 hr (data not shown). RT-PCR detected COX-2 mRNA in human pancreatic islets cultured in 0.2% FBS (Fig. 2B); these levels were increased by 3-hr treatment with IL-1 (5 ng/ml). Pretreatment with dexamethasone (1 μM) for 3 hr suppressed levels of COX-2 mRNA detected in human pancreatic islets cultured either with 0.2% FBS or 0.2% FBS plus IL-1 for 2 hr.
Figure 1.
Northern analysis of poly(A) mRNA for COX-1 and COX-2 in HIT-T15 (H; lanes 1–3) cells grown in RPMI 1640 medium containing 10% FBS. Twenty-eight hours before harvesting cells for RNA extraction, FBS was removed from the medium. Cells were incubated in media with 0%, 0.2%, or 10% FBS for 4 hr immediately before harvesting. Total RNA was extracted and poly(A) mRNA was isolated. Six micrograms of poly(A) mRNA from HIT cells were loaded per lane, electrophoresed in a 1.5% agarose-formaldehyde gel, and transferred to a nylon membrane by electroblotting. Poly(A) mRNA was used for these experiments because the size of COX-2 mRNA is close to the size of 28 S ribosomal RNA. Twenty micrograms of total RNA from 3T3 cells also is shown (lane 4) as verification that the COX-1 probe was reliable in an even less mRNA-enriched sample. These results are representative of identical results from three separate experiments and demonstrate the absence of COX-1 mRNA under basal (0.2% FBS) or stimulated (10% FBS) conditions (A). In contrast, COX-2 mRNA was readily detectable under basal and stimulated conditions (B). The probe used for Northern analysis readily detected COX-1 mRNA under basal conditions in 3T3 cells. COX/β-actin mRNA ratios for lanes 1–7 were 0.02, 0.09, 0.12, 3.62, 0.78, 0.74, and 1.46, respectively.
Figure 2.
(A) RT-PCR for COX-1 and COX-2 expression in Syrian hamster tissues. Total RNA (2 μg) was extracted from 3T3 and HIT-T15 cells cultured with 10% FBS and from Syrian hamster islets, spleen, kidney, and liver. Oligonucleotide primers were synthesized based on the published consensus sequence of mouse, rat, and sheep. Single-stranded cDNA transcribed from total RNA was used for PCR amplification with COX-1, COX-2, and TFIID-specific primers. Amplified cDNAs were analyzed by 6% nondenaturing PAGE with visualization by silver staining. Detection of COX-1 mRNA in HIT cells and Syrian hamster islets was barely detectable by using RT-PCR whereas COX-1 mRNA was readily detectable in 3T3 cells and Syrian hamster spleen, kidney, and liver in this and in two other separate experiments. In contrast, RT-PCR readily demonstrated COX-2 mRNA in HIT cells and hamster islets whereas smaller amounts were observed in the other tissues. COX-1/TFIID ratios for lanes 1–6 were 4.23, 0.29, 0.33, 0.64, 0.65, and 0.61, respectively. COX-2/TFIID ratios for lanes 1–6 were 2.62, 2.14, 2.60, 1.30, 1.09, and 0.39, respectively. (B) RT-PCR for COX-2 expression in human pancreatic islets. Human islets were cultured in RPMI 1640 medium/0.2% FBS for 18 hr and then incubated for 3 hr in one of the following conditions: control, 1 μM dexamethasone, 5 ng/ml IL-1, or dexamethasone followed by an additional incubation for 2 hr with 5 ng/ml IL-1. Total RNA (2 μg) was isolated, reverse-transcribed to cDNA, and amplified with specific human COX-2 and TFIID primers. The RT-PCR products were detected by 6% nondenaturing PAGE and visualized by silver staining. RT-PCR readily detected COX-2 mRNA in human pancreatic islets cultured in media containing 0.2% FBS in this and in two other separate experiments. COX-2 mRNA levels also increased when islets were cultured in media containing 0.2% FBS and IL-1. Pretreatment with dexamethasone suppressed levels of COX-2 mRNA cultured either with 0.2% FBS or IL-1 present in the media. COX-2/TFIID ratios for lanes 1–4 were 1.27, 0.16, 1.75, and 0.37, respectively.
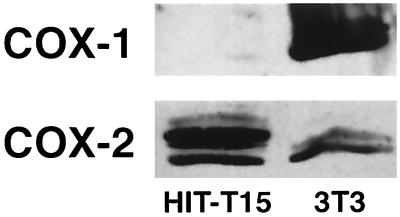
COX-2 Protein and PGE2.
COX-2, but not COX-1, protein was detectable in HIT-T15 cells (Fig. 3). IL-1 increased PGE2 production of PGE2 by HIT-T15 cells over 24 hr, and this effect was inhibited when the specific COX-2 inhibitor NS-398 (0.01 mM) was included in the incubations (Table 1). Both control and IL-1-treated cells were >90% viable by trypan blue exclusion at 24 hr.
Figure 3.
Western analysis for COX-1 and COX-2 using HIT-T15 cells. Adequacy of the COX-1 antisera was demonstrated in control studies with 3T3 cells.
Table 1.
Basal and IL-1 (5 ng/ml) − stimulated PGE2 concentrations (pg/ml) in media during HIT-T15 cell incubations in RMPI 1640 containing 0.2% FBS in absence and presence of NS-398 (0.01 mM)
| Hours | Control | IL-1 | NS-398 | IL-1 with NS-398 |
|---|---|---|---|---|
| 2 | 100 ± 5 | 240 ± 5** | <20 | <20 |
| 4 | 210 ± 80 | 320 ± 30* | <20 | <20 |
| 8 | 340 ± 30 | 380 ± 25* | <20 | <20 |
| 24 | 475 ± 120 | 870 ± 220** | <20 | <20 |
Levels greater than control ∗, P < .005; ∗∗, P < .0005. n = 4 experiments.
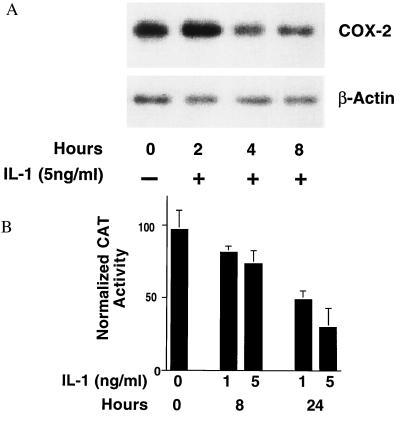
Time Course of IL-1 Effects on COX-2 mRNA and COX-2 Promoter Activity.
Northern blot analysis was performed on total RNA isolated from HIT-T15 cells exposed to 5 ng/ml of IL-1 for various periods of time (Fig. 4A). A biphasic pattern of COX-2 mRNA expression was observed. An initial, transient increase in COX-2 mRNA, peaking 2 hr after exposure to IL-1, was reproducibly observed. This increase was followed by a progressive decline to levels approximately 30% of baseline. A transient transfection reporter gene assay was used to examine the effects of IL-1 on COX-2 promoter activity in HIT cells. The proximal 2,600 bp of the COX-2 promoter (−2,698/+32) were used to drive the expression of a CAT reporter gene. Consistent with the baseline expression of COX-2 mRNA, readily detectable levels of COX-2 promoter activity were present in HIT cells cultured in low-serum media (Fig. 4B). Inclusion of IL-1 in the media resulted in a dose- and time-dependent decrease in COX-2 promoter activity. Although it was not technically feasible to measure CAT activity before 8 hr, by 24 hr COX-2 promoter activity was reduced to levels 70% below baseline, corresponding quantitatively with the decrease observed in mRNA levels at that time.
Figure 4.
(A) Northern blot analysis of total RNA from HIT-T15 cells exposed to 5 ng/ml IL-1 for the indicated periods of time. HIT cells were subcultured overnight in RPMI with 0.2% FBS to obtain a quiescent state before exposure to IL-1. Fifteen milligrams of RNA was electrophoresed in a 1.5% agarose-formaldehyde gel and transferred to a nylon membrane by electroblotting. Blots were probed sequentially with probes specific for COX-2 and beta-actin and are representative of three experiments. COX-2/beta-actin ratios for time points 0, 2, 4, and 8 hr were 4.2, 7.1, 3.8, and 2.3, respectively. Mean ± SE of the three experiments were 4.5 ± 1.7, 8.1 ± 1.7, 2.3 ± 1.4, and 1.8 ± 1.0, respectively. No COX-1 was observed by Northern analysis of HIT cell RNA under control conditions nor after exposure to IL-1. (B) Effect of IL-1 on COX-2 promoter activity in HIT cells. Transient transfection of HIT-T15 cells with a CAT reporter gene construct containing base pairs (−2,698/+32) of the murine COX-2 promoter (COX-2/CAT) was performed in the presence or absence of IL-1 for the indicated periods of time. HIT cells (1.2 × 106) were subcultured overnight in RPMI with 0.2% FBS and then transfected with purified plasmid (either 1.0 μg of COX-2/CAT or 0.5 μg of Rous sarcoma virus/CAT) by using a lipofectin technique. After transfection, cells were exposed to experimental conditions as indicated. Data were expressed as percentage of [14C]chloramphenicol converted to acetylated chloramphenicol per microgram of soluble protein and then normalized to Rous sarcoma virus/CAT activity to correct for variations in transfection efficiency. Results are the mean ± SE of two experiments each run in triplicate.
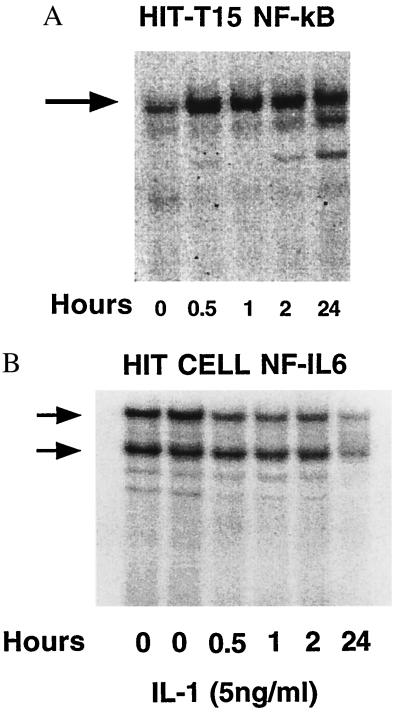
NF-κB and NF-IL-6.
NF-κB binding activity was observed in HIT cells (Fig. 5A), In data not shown, binding was observed in rat islets and human islets, competed for by unlabeled oligodeooxynucleotide, and supershifted by antiserum specific for individual members of the NF-κB family of nuclear proteins. Low levels of nuclear NF-κB binding were observed under low-serum culture conditions; IL-1 induced an increase in NF-κB binding in HIT cells and islets persisting up to 24 hr. EMSA analysis of NF-IL-6 binding revealed the opposite pattern. NF-IL-6 binding to the COX-2 promoter was maximal before exposure to IL-1 and exposure to IL-1 resulted in a time-dependent decrease in NF-IL-6 binding (Fig. 5B). By 24 hr, NF-IL-6 activity had decreased by approximately 70% from baseline levels. NF-IL-6 binding was competed for by unlabeled oligodeooxynucleotide and supershifted by antiserum specific for NF-IL-6. No supershifting was observed with antiserum specific for another member of the C/EBP family, C/EBP-α (data not shown).
Figure 5.
(A) EMSA of binding of nuclear protein to the NF-κB region of the COX-2 promoter. HIT-T15 cells were exposed to 5 ng/ml of IL-1 for the indicated periods of time. The NF-κB complex increased with IL-1 exposure in this and two other experiments. (B) EMSA of binding of nuclear protein to the NF-IL-6 region of the COX-2 promoter. HIT-T15 cells were exposed to 5 ng/ml of IL-1 for the indicated periods of time. The two lanes labeled 0 hr under HIT cells represent subculturing of cells in both the standard quiescent condition (0.2% FBS, lane 1) as well as in elevated serum conditions (10% FBS, lane 2). The NF-IL-6 complex decreased with IL-1 exposure in this and two other experiments.
Mutagenesis of the NF-κB and NF-IL-6 Binding Sites in the COX-2 Promoter.
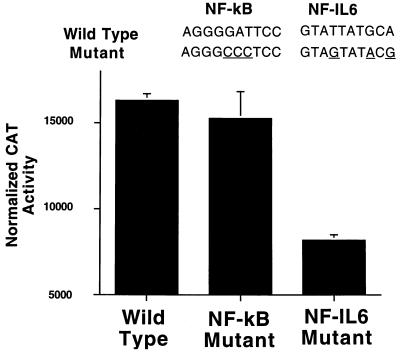
To determine whether the readily detectable levels of COX-2 promoter activity were directly related to increased levels of NF-IL-6 activity in beta cells, mutations were introduced into the putative binding sites of both NF-κB and NF-IL-6 within the COX-2 promoter. Mutated promoters driving a CAT reporter gene were transiently transfected into HIT cells, and promoter activity was assessed under 0.2% FBS conditions (Fig. 6). Specific mutation of the NF-IL-6 binding site resulted in a 50% reduction in promoter activity, whereas mutation of the NF-κB binding site had no significant effect.
Figure 6.
Effect of selective mutation of the NF-IL-6 site on COX-2 promoter activity. Nonrecognition of the mutated sequence was confirmed by demonstrating an inability of the double-stranded mutant sequence to compete with wild-type sequences in EMSA analysis at a 100-fold excess (data not shown). HIT cells were subcultured in RPMI with 0.2% FBS overnight before transfection. After transfection the cells were maintained in RPMI with 0.2% FBS (basal conditions) for the 24-hr period of reporter gene expression. Data are expressed as percentage of [14C]chloramphenicol converted to acetylated chloramphenicol per microgram of soluble protein and then normalized to Rous sarcoma virus/CAT activity to correct for variations in transfection efficiency. Results are the mean ± SE of three experiments each run in triplicate.
DISCUSSION
These findings demonstrate that COX-2, and not COX-1, gene expression is dominant in pancreatic islet tissue under both basal and stimulated conditions. This dominance was documented by using Northern and RT-PCR analysis of HIT-T15 cells, Syrian hamster islets, and human pancreatic islets. In contrast, Syrian hamster spleen, kidney, and liver under basal conditions demonstrated the expected predominance of COX-1 gene expression relative to COX-2 gene expression. Preactivation of islet tissue by exposure to stimulatory agents contained within collagenase and Ficoll cannot be an explanation because identical treatment of hepatocytes failed to induce COX-2 gene expression. IL-1 stimulation of COX-2 gene expression in human islets was appropriately suppressed by dexamethasone. Nearly total inhibition of PGE2 synthesis by a COX-2-specific inhibitor (NS-398) confirmed the importance of COX-2 expression under both basal and IL-1-stimulated conditions. IL-1 regulation of COX-2 mRNA levels was biphasic. The bimodal nature of regulation of IL-1 on COX-2 mRNA levels we observed is similar to the pattern and kinetics of other IL-1-responsive beta cell functions, including insulin content and release (11), mitochondrial oxidative activity (12), calcium uptake (13), and phosphoinositide metabolism (14). After an initial, small, but reproducible, increase in COX-2 mRNA lasting 2–4 hr, a more dramatic decrease in mRNA expression was observed extending to 24 hr. This decrease in COX-2 mRNA expression after 24 hr of IL-1 exposure corresponded quantitatively and temporally with a decrease in COX-2 promoter activity during the same time interval. These findings do not contradict the finding of increased PGE2 levels 24 hr after IL-1 exposure because the half-life of COX-2 mRNA generally is shorter than that of PGE2 and the observed PGE2 levels represent PGE2 made by the cells and accumulated in the media over 24 hr.
Consensus binding sites for NF-κB and NF-IL-6 are present within the COX-2 promoter of human, rat, and mouse, and these factors are known to regulate IL-1-induced transcriptional activity (30). Flodström et al. (31) previously have reported IL-1 induction of NF-κB activity in human islets (31). In our studies, EMSA of nuclear extracts from HIT cells, rat islets, and human islets demonstrated specific NF-κB complexes. Low baseline levels of NF-κB binding to the COX-2 promoter were dramatically increased after exposure to IL-1 and remained elevated for 24 hr. This increase in NF-κB corresponds well with the early, transient induction of both COX-2 mRNA and PGE2 production. More importantly, however, our studies demonstrated that NF-IL-6 was expressed prominently within beta cells basally before exposure to IL-1, a finding not caused by activation by serum factors because equivalent levels of NF-IL-6 binding were observed in both high and low FBS concentrations. IL-1 caused a time-dependent decrease in NF-IL-6 binding. Both of these findings, the high basal expression and subsequent reduction in binding after IL-1 exposure, raise the possibility that NF-IL-6 may play at least a partial role in the expression pattern of COX-2 we observed. That specific mutation of the NF-IL-6 binding motif within the COX-2 promoter reduced basal promoter activity by 50% supports this possibility. In contrast, mutational studies of the NF-κB binding site indicated that this nuclear factor does not contribute significantly to basal COX-2 gene expression. In any event, these experiments provide detection of intraislet NF-IL-6 and its surprisingly high levels in the basal state.
Only a few studies of COX-1 and COX-2 gene expression in pancreatic islets and islet cell lines have been reported previously. Whole pancreas, which is comprised of 97% nonislet cells, from human trauma victims has been examined by RT-PCR, which detected both COX-1 and COX-2 expression (32). However, no attempts were made to examine islets specifically nor was the issue of basal vs. stimulated COX-2 gene expression in tissue from these stressed humans addressed. Corbett et al. (19) reported IL-1 induction of COX-2 gene expression in rat islets, but did not compare the relative abundance of COX-1 and COX-2 mRNAs. Our finding of relative dominance of COX-2 gene expression in the basal state contrasts with most reports using other cells (33–35). Nonetheless, in varying degrees, support for our observations can be found. Yamagata et al. (36) observed by Northern analysis of total RNA from rat brain a very low level of COX-2, but not COX-1, mRNA in hippocampal and cortical tissue that was greatly increased by electroconvulsive seizures. Our findings differ from those of Yamagata et al. in that we detected a much greater level of COX-2 mRNA in unstimulated islets and show that this level was increased by IL-1 and suppressed by dexamethasone. Additionally, to be certain of our findings, we used poly(A) mRNA, rather than total RNA, for Northern analysis to avoid potential confusion with ribosomal 28S, which appears very close to, and sometimes overlaps with, COX-2 mRNA. Harris et al. (37), studying kidney tissue by in situ hybridization and Western analysis, detected renal cortical COX-2 expression in macula densa of the juxaglomerular apparatus and in adjacent epithelial cells of the cortical thick ascending limb of Henle. Asano et al. (38) reported dominant COX-2, rather than COX-1, gene expression in normal human bronchial epithelial cell cultures, but not in bronchial smooth muscle cell cultures, grown in media containing 2% FBS. Most recently, Narko et al. (39) reported COX-2 mRNA detectability in freshly isolated granulosa cells, but not in cultured in cultured granulosa-luteal cells.
That islet beta cells dominantly express COX-2 either at rest or when stimulated may represent more than an organelle-specific curiosity. Cytokines, such as IL-1, stimulate eicosanoid synthesis predominantly through the activation of COX-2 gene expression. Consequently, dominance in the basal state of islet of COX-2 mRNA, protein synthesis, and enzymatic activity heightens the probability of IL-1-stimulated PGE2 production. The clinical relevance of such a scenario is presently not clear as PGE2 in some tissues is proinflammatory whereas in others is cytoprotective. The extent to which PGE2 has either or both effects was not examined in this work. In earlier studies (16, 17) we observed PGE2 inhibits glucose-induced insulin secretion; however, even this phenomenon need not necessarily be deleterious because it is reversible and theoretically could be advantageous in protecting the islet from excessive work during stress-induced hyperglycemia. The answers to such questions about beta cell function await completion of studies extensively examining concentration-response and duration-response curves for IL-1 and PGE2 under varying glucose concentrations in the presence and absence of specific and structurally dissimilar COX-2 inhibitors.
Acknowledgments
We gratefully acknowledge the technical assistance of Ms. Elizabeth Oseid, statistical assistance from Will Thomas, Ph.D., and the secretarial services of Ms. Lucy Mittag. This work was supported by National Institutes of Health Grant R0I-DK-38325 (R.P.R.) and the Alberta Foundation for Diabetes Research (R.V.R.)
ABBREVIATIONS
- COX
cyclooxygenase
- IL
interleukin
- NF
nuclear factor
- FBS
fetal bovine serum
- RT-PCR
reverse transcriptase–PCR
- EMSA
electrophoretic mobility shift assay
- CAT
chloramphenicol acetyltransferase
- PEG
polyethyleneglycol
- TFIID
transcription factor II D
- PGE2
prostaglandin E2
References
- 1.Rosen G D, Birkenmeier T M, Raz A, Holtzman M J. Biochem Biophys Res Comm. 1989;164:1358–1365. doi: 10.1016/0006-291x(89)91819-6. [DOI] [PubMed] [Google Scholar]
- 2.Fu J-Y, Masferrer J L, Seibert K, Raz A, Needleman P. J Biol Chem. 1990;28:16737–16740. [PubMed] [Google Scholar]
- 3.Crofford L J, Wilder R L, Ristimaki A P, Sano H, Remmers E F, Epps H R, Hla T. J Clin Invest. 1994;93:1095–1101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meade E A, Smith W L, DeWitt D L. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 5.Mitchell J A, Akarasereenont P, Thiemermann C, Flower R J, Vane J R. Proc Natl Acad Sci USA. 1994;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie W, Merrill J R, Simmons D L, Bradshaw W S. Arch Biochem Biophys. 1993;300:247–252. doi: 10.1006/abbi.1993.1034. [DOI] [PubMed] [Google Scholar]
- 7.Leung K, Betts J C, Xu K, Nabel G J. J Biol Chem. 1994;269:1579–1582. [PubMed] [Google Scholar]
- 8.Isshiki H, Akira S, Tanabe O, Nakajima T, Shimamoto T, Hirano T, Kishimoto T. Mol Cell Biol. 1990;10:2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helqvist S. Dan Med Bull. 1994;41:151–166. [PubMed] [Google Scholar]
- 10.Mandrup-Poulsen T. Diabetologia. 1996;39:1005–1029. doi: 10.1007/BF00400649. [DOI] [PubMed] [Google Scholar]
- 11.Spinas G A, Palmer J P, Mandrup-Poulsen T, Andersen H, Nielsen J H, Nerup J. Acta Endocrinol. 1988;119:307–311. doi: 10.1530/acta.0.1190307. [DOI] [PubMed] [Google Scholar]
- 12.Eizirik D L, Sandler S. Diabetologia. 1989;32:769–773. doi: 10.1007/BF00264905. [DOI] [PubMed] [Google Scholar]
- 13.Borg L A H, Sandler S, Eizirik D L. Immunol Lett. 1990;26:253–258. doi: 10.1016/0165-2478(90)90155-j. [DOI] [PubMed] [Google Scholar]
- 14.Zawalich W S, Zawalich K C, Rasmussen H. Endocrinology. 1989;124:2350–2357. doi: 10.1210/endo-124-5-2350. [DOI] [PubMed] [Google Scholar]
- 15.Meredith M, Rabaglia M E, Corbett J A, Metz S A. Diabetes. 1996;45:1783–1791. doi: 10.2337/diab.45.12.1783. [DOI] [PubMed] [Google Scholar]
- 16.Robertson R P. Diabetes Metab Rev. 1986;2:261–296. doi: 10.1002/dmr.5610020304. [DOI] [PubMed] [Google Scholar]
- 17.Robertson R P, Chen M. J Clin Invest. 1977;60:747–753. doi: 10.1172/JCI108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowluru A, Metz S A. Biochem J. 1994;297:399–406. doi: 10.1042/bj2970399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbett J A, Kwon G, Turk J, McDaniel M L. Biochemistry. 1993;32:13767–13770. doi: 10.1021/bi00213a002. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovitch A, Suarez-Pinzon W, El-Sheikh A, Sorensen O, Power R F. Diabetes. 1996;45:749–754. doi: 10.2337/diab.45.6.749. [DOI] [PubMed] [Google Scholar]
- 21.Eizirik D L, Flodström M, Karlsen A E, Welsh N. Diabetologia. 1996;39:875–890. doi: 10.1007/BF00403906. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H-J, Walseth T F, Robertson R P. Diabetes. 1989;38:44–48. doi: 10.2337/diab.38.1.44. [DOI] [PubMed] [Google Scholar]
- 23.Lacy P, Kostianovsky M. Diabetes. 1969;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 24.Warnock G L, Knetemann N M, Rajotte R V. In: The Endocrine Pancreas. Samols E, editor. New York: Raven; 1991. pp. 487–517. [Google Scholar]
- 25.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Bassam B J, Caetano-Anollés G, Gresshoff P M. Anal Biochem. 1991;196:80–88. doi: 10.1016/0003-2697(91)90120-i. [DOI] [PubMed] [Google Scholar]
- 27.Sirois J, Levy L O, Simmons D L, Richards J S. J Biol Chem. 1993;16:12199–12206. [PubMed] [Google Scholar]
- 28.Seaquist E R, Robertson A N, Shoger K D, Walseth T F, Robertson R P. Diabetes. 1992;41:1390–1399. doi: 10.2337/diab.41.11.1390. [DOI] [PubMed] [Google Scholar]
- 29.Habib A, Créminon C, Frobert Y, Grassi J, Pradelles P, Maclouf J. J Biol Chem. 1993;268:23448–23454. [PubMed] [Google Scholar]
- 30.Smith W L, DeWitt D L. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 31.Flodström M, Welsh N, Eizirik D L. FEBS Lett. 1996;385:4–6. doi: 10.1016/0014-5793(96)00337-7. [DOI] [PubMed] [Google Scholar]
- 32.O’Neill G P, Ford-Hutchinson A W. FEBS Lett. 1993;330:156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- 33.Smith W L, DeWitt D L. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 34.Robertson R P. Trends Endocrinol Metab. 1995;6:293–297. doi: 10.1016/1043-2760(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 35.Herschman H R. Biochim Biophys Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 36.Yamagata K, Andreasson K I, Kaufmann W E, Barnes C A, Worley P F. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 37.Harris R C, McKanna J A, Akai Y, Jacobson H R, Dubois R N. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asano K, Lilly C M, Drazen J M. Am J Physiol. 1996;271:L126–L131. doi: 10.1152/ajplung.1996.271.1.L126. [DOI] [PubMed] [Google Scholar]
- 39.Narko N, Ritvos O, Ristimaki A. Endocrinology. 1997;138:3638–3644. doi: 10.1210/endo.138.9.5388. [DOI] [PubMed] [Google Scholar]