Abstract
Stromal cell-derived factor 1 alpha (SDF-1α) and its receptor CXCR4 play important roles in the pathogenesis of human immunodeficiency virus type one (HIV-1)-associated dementia (HAD) by serving as a HIV-1 co-receptor and affecting cell migration, virus-mediated neurotoxicity, and neurodegeneration. However, the underlying mechanisms regulating SDF-1 production during disease are not completely understood. In this report we investigated the role of HIV-1 infected and immune competent macrophage, the principal target cell and mediator of neuronal injury and death in HAD, in regulating SDF-1 production by astrocytes. Our data demonstrated that astrocytes are the primary cell type expressing SDF-1 in the brain. Immune-activated or HTV-1-infected human monocyte-derived-macrophage (MDM) conditioned media (MCM) induced a substantial increase in SDF-1 production by human astrocytes. This SDF-1 production was directly dependent on MDM IL-1β following both viral and immune activation. The MCM-induced production of SDF-1 was prevented by IL-1β receptor antagonist (IL-1Ra) and IL-1β siRNA treatment of human MDM. These laboratory observations were confirmed in severe combined immunodeficient (SCID) mice with HIV-1 encephalitis (HIVE). In these HIVE mice, reactive astrocytes showed a significant increase in SDF-1 expression, as observed by immunocytochemical staining. Similarly, SDF-1 mRNA levels were increased in the encephalitic region as measured by real time RT-PCR, and correlated with IL-1β mRNA expression. These observations provide direct evidence that IL-1β, produced from HIV-1-infected and/or immune competent macrophage, induces production of SDF-1 by astrocytes, and as such contribute to ongoing SDF-1 mediated CNS regulation during HAD.
Keywords: SDF-1, HIV-1-associated dementia, IL-1β, astrocytes, macrophages
INTRODUCTION
Stromal cell-derived factor 1 (SDF-1), the ligand for CXCR4, is a CXC chemokine that elicits cell chemotaxis (Aiuti et al., 1997; Lazarini et al., 2003; Nagasawa et al., 1996; Peng et al., 2004). Two isoforms (SDF-1α and SDF-1β) are encoded by a single gene and arise from alternative splicing (Shirozu et al., 1995). Both SDF-1 and CXCR4 are essential for development (Lu et al., 2002; Ma et al., 1998; Zou et al., 1998). In the brain, SDF-1 is a potent chemoattractant for neural stem cells and affects neural stem cell and astrocyte proliferation. This chemotaxis is critical to the patterning of neurons or glial cells (Bagri et al., 2002; Bajetto et al., 2001a; Lu et al., 2002; Peng et al., 2004; Reiss et al., 2002; Stumm et al., 2003). The abundant and constitutive CNS expression of SDF-1 and CXCR4 further support their roles in CNS homeostasis (Bajetto et al., 2001b; Tham et al., 2001).
HIV-1-associated dementia (HAD) is a neurodegenerative disease clinically manifested by progressive cognitive, motor, and behavioral abnormalities (McArthur et al., 2003). HAD, a chronic inflammatory state within the CNS, influences chemokine and chemokine receptor expression (Cota et al., 2000). An estimated 9–11% of HIV-infected individuals develop significant cognitive dysfunction despite the introduction of potent antiretroviral therapy (ART) (Maschke et al., 2000). HIV encephalitis (HIVE), the pathological correlate of HAD, is characterized by reactive astrogliosis, accumulation of activated macrophages, microglial activation, virus-infected multi-nucleated giant cells, and neuronal damage (Glass et al., 1993; Navia et al., 1986; Persidsky et al., 1996; Tyor et al., 1992). In HAD and HIVE, mononuclear phagocytes (MP; perivascular macrophages and microglia) are the principal productively infected cells in the brain. Following immune activation or HIV-1 infection, MP undergo functional alterations that lead to secretion of bioactive products, including cytokines, chemokines, viral proteins, and neurotoxins, that are either directly involved in neuronal injury or indirectly by inducing astrocyte dysfunction and microglia activation (Conant et al., 1998; Gabuzda et al., 1998; Gendelman et al., 1997; Glass et al., 1995; He et al., 1997; Kaul et al., 2001; Kolson and Gonzalez-Scarano, 2000; Lipton and Gendelman, 1995; Masliah et al., 1997; Nath and Geiger, 1998; Strizki et al., 1996).
SDF-1 expression has been shown to be increased in patients with HIVE (Langford et al., 2002; Rostasy et al., 2003). Recent reports demonstrate that CXCR4, a co-receptor for HIV-1 infection, plays an important role in virus-mediated neuronal injury during HAD (Allen and Attwell, 2001; Bezzi et al., 2001; Hesselgesser et al., 1998; Kaul and Lipton, 1999; Meucci et al., 1998; Zhang et al., 2003; Zheng et al., 1999). However, the source and regulatory mechanisms of SDF-1 production during HAD remained undefined. We have previously shown that SDF-1 mRNA levels are increased in astrocytes exposed to HIV-1-infected or immune-activated macrophage conditioned media (MCM) (Zheng et al., 1999), indicating that MP-astrocyte interactions play an essential role in SDF-1 regulation. However, the individual factors that stimulate SDF-1 production by astrocytes have not been identified.
In the current report, we demonstrate that SDF-1 is predominantly produced by astrocytes as compared to neurons, macrophages, and neural progenitor cells. Astrocyte cultures treated with supernatants from immune-activated and HIV-1-infected monocyte-derived macrophages (MDM) showed an increase in SDF-1 production. IL-1 receptor antagonist (IL-1Ra) and IL-1β siRNA abolished this effect. In SCID mice with HIVE, human HIV-1 infected MDM induced an increase in astrocyte SDF-1 expression. Further, SDF-1 mRNA level was increased and correlated with IL-1β mRNA expression. These data demonstrate that IL-1β, produced from HIV-1 infected and activated macrophages, is a principal inducer for SDF-1 production in astrocytes.
MATERIALS AND METHODS
Monocyte Cell Culture and HIV-1 Infection
Human monocytes were recovered from peripheral blood mononuclear cells (PBMCs) of HIV-1,2 and hepatitis B seronegative donors after leukopheresis and counter current centrifugal elutriation (Gendelman et al., 1988). Monocytes were cultured as adherent monolayers at a density of 1.1 × 106 cells/well in 24-well plates and cultivated in Dulbecco’s modified Eagles medium (DMEM; GIBCO Invitrogen Corp, Carlsbad, CA) with 10% heat-inactivated pooled human serum (Cambrex Bio Science, Walkersville, MD), 50 μg/mL gentamicin or 10 μg/mL ciprofloxacin (Sigma-Aldrich, St. Louis, IL), and 1,000 U/mL highly purified recombinant human macrophage colony stimulating factor (MCSF) (a generous gift from Wyeth Institute, Cambridge, MA).
Seven days after plating MDM were infected with HIV-1 strains ADA, BAL, or JR-FL at a multiplicity of infection (MOI) of 0.1 virus/target cell (Zheng et al., 2001). Four to five days after infection HIV-1-infected and replicate uninfected MDM were treated with/without lipopolysaccharide (LPS) (Sigma, 0.1 μg/mL) for 3 h. Cells were then rinsed two times with DMEM to remove residual LPS and serum free neurobasal media was placed onto MDM for 24 h. Culture supernatants were obtained and subsequently stored at −80°C until assay.
siRNA Transfection
Pre-designed siRNA duplexes targeted against IL-1β mRNA were synthesized by Ambion Inc. (Austin, Texas). Three to four days post-infection, MDM were transfected with 100 nM siRNA duplex for 24–48 h in the presence of siIMPORTER (Upstate Cell Signaling Solutions, Charlottesville, VI) according to the manufacturer’s instructions. A non-specific control siRNA (Dharmacon, Lafayette, CO) was also transfected at the same concentration as control. To evaluate transfection efficiency, control and HIV-1-infected MDM were transfected with control siRNA siGLO (red fluorescence tagged siRNA) (Dharmacon). At 24 h post transfection, cells were incubated with Hoechst 33342 (1:5,000, Sigma) for nuclear staining; transfected and total cells were counted.
Human Fetal Neuron, Neural Progenitor Cell, and Astrocyte Culture
Human fetal astrocytes, neurons, and neural progenitor cells were isolated from human fetal brain tissue (gestational age 13–16 weeks) from elective aborted specimens in full compliance with the University of Nebraska Medical Center (UNMC) and National Institutes of health (NIH) ethical guidelines. Human fetal cortical neurons (HN) were prepared as previously described, with modification (Zheng et al., 1999). Briefly, dissociated brain tissue was incubated with 0.25% trypsin for 30 min, followed by neutralization with 10% FBS, and further dissociated by trituration. The single-cell suspension was cultured on poly-D-lysine coated plates in neuralbasal media supplemented with B27 (Invitrogen). This procedure typically results in 70% enriched neurons as assessed by immunostaining with anti-MAP-2 antibody (Chemicon, Temecula, CA).
Human neural progenitor cell (NPC) cultures were isolated from human brain tissue as previously described (Peng et al., 2004). Briefly, NPC were cultured in substrate-free tissue culture flasks and grown as spheres in neurosphere initiation medium (NPIM), which consisted of X-Vivo 15 (BioWhittaker, Walkersville, ME) with N2 supplement (Invitrogen), basic fibroblast growth factor (FGF, 20 ng/mL, Sigma-Aldrich), epidermal growth factor (EGF, 20 ng/mL, Sigma-Aldrich), leukemia inhibitory factor (LIF, 10 ng/mL, Chemicon), neural cell survival factor-1 (NSF-1, Cambrex Bio Science), and 60 ng/mL N-acetyl-cysteine (Sigma-Aldrich) (Uchida et al., 2000). Cells were passaged at 2-week intervals as previously described (Peng et al., 2004). Immunostaining studies showed that >90% cells were positive for nestin, a neural stem cell marker.
Human astrocytes were isolated from fetal brain tissue cortexes as previously described (Ghorpade et al., 2003). Cells were cultured at a density of 2 × 107 cells/150 cm2 in DMEM/F12 (Invitrogen), supplemented with fetal bovine serum (FBS, 10%; GIBCO Invitrogen Corp), and an antibiotic mixture containing penicillin, streptomycin, and neomycin (Invitrogen). The adherent astrocytes were treated with 0.25% trypsin after 2 weeks in culture and the cell suspension was cultured under the same conditions to enhance purity. Astrocyte preparations were assessed by immunocytochemical staining using antibodies to glial fibrillary acidic protein (GFAP, Dako Corp., Carpinteria, CA). This process yields a culture of >98% pure astrocytes.
Human astrocytes were seeded into a 48-well plate at a density of 1.5 × 105 cells/well. Cells were treated with 25% MDM conditioned media (MCM) or IL-1β (10 ng/mL, R&D Systems, Minneapolis, MN), IFN-γ (100 ng/mL, R&D Systems), TNF-α (50 ng/mL, R&D Systems), and HIV-1 gp120 (1 nM, Advanced Biotechnologies Incorporated, Columbia, MD) for 48 h. To compete with IL-1β for receptor binding, cells were pre-treated with IL-1 receptor antagonist (IL-1Ra recombinant protein, 100 ng/mL; R&D Systems) for 1 h before IL-1β treatment. The supernatants were collected and assayed for SDF-1 production by Fluro ELISA.
RNA Extraction and TaqMan Real-Time RT-PCR
Total RNA was isolated with TRIzol Reagent (Invitrogen) and RNeasy Mini Kit (QIAGEN Inc., Valencia, CA). Assays-on-Demand primers for human IL-1β (ID No., Hs00174097_ml), mouse IL-1β (ID No., Mm00434228_ml), human SDF-1 (ID No., Hs00171022_ml), mouse SDF-1 (ID No., Mm00445552_ml), human GAPDH (ID No., 4310884E), and mouse GAPDH (ID No., Mm99999915_gl) were purchased from Applied Biosystems Inc (Foster City, CA). Real-time-reverse-transcription polymerase chain reaction (RT-PCR) was carried out using the one-step quantitative TaqMan Real-time RT-PCR system (Applied Biosystems Inc.). IL-1β and SDF-1α mRNA levels were determined and standardized with GAPDH internal control.
ELISA
Supernatants from MDM were collected for IL-1β determination by an in house ELISA (for paired antibodies, R&D Systems, Minneapolis, MN) as described (Erichsen et al., 2003). To assess concentrations of secreted SDF-1 in supernatants of cytokine- or MCM-treated cells, we developed a sandwich fluorescence (Fluro) ELISA system with modifications to a previously described assay (Jefcoat et al., 2001). In brief, 96-well microtiter plates (Costar) were coated overnight at room temperature with a mouse anti-SDF-1 monoclonal antibody (4 μg/mL, R&D Systems) in PBS. Non-specific binding was blocked for 2 h with 1% BSA in PBS. Triplicate samples of cell supernatants (100 μL) or a serial dilution of standards of human recombinant SDF-1α (R&D Systems) were applied to the wells and incubated overnight at 4°C. Samples were then incubated for 1 h at room temperature with the biotinylated goat anti-SDF-1 antibody (300 ng/mL), followed by 1 h incubation with HRP-conjugated streptavidin (R&D Systems). After three washes with PBS containing 0.05% Tween 20 (PBST), the final reaction product was detected using QuantaBlu™ Fluorogenic Peroxidase Substrate (PIERCE, Rockford, IL). The plate was read by SpectraMax GEMINI (325 nm excitation, 420 nm emission) (Molecular Devices). The sensitivity for this assay is 100 pg/mL for SDF-1.
Severe Combined Immunodeficient HIVE Mice
Four-week-old male C.B.-17-SCID mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were maintained in sterile microisolator cages under pathogen-free conditions in the Laboratory of Animal Medicine at UNMC in accordance with ethical guidelines for care of laboratory animals set forth by the National Institutes of Health. One day after infection, HIV-1ADA-infected MDM (5 × 105 cells in 5 μL) were injected intracranially by stereotactic methods (Persidsky et al., 1996). Replicate SCID mice received intracranial injections of media (sham-operated) and served as controls. Four animals were included in each group. Seven days after MDM injection, mice were killed and CNS tissue was collected and subjected to immunocytochemical staining. An additional 10 mice were injected with HIV-1-infected MDM and RNA was extracted from 2 mm brain tissue for real time RT-PCR.
Histopathology and Image Analysis
Mouse brain tissue was collected at necropsy, fixed in 4% phosphate-buffered paraformaldehyde, and embedded in paraffin. Paraffin blocks were cut to identify the injection site. Sixty to 120 serial (5-μm-thick) sections were cut from the injection site and 3–7 sections (10 sections apart) were analyzed. Antibody to HIV-1 p24 antigen (Dako Corp.) was used to test for virus-infected human MDM. Antibodies to vimentin intermediate filaments (clone Vim 3B4, Dako Corp.) were used for detection of human cells. Mouse astrocytes were detected by antibody for GFAP; neurons were detected by antibody for MAP-2 (Chemicon, Temecula, CA). SDF-1 expression was detected by antibody for SDF-1 (R&D). Double-immunofluorescence staining was performed using Alexa Fluor 488 (green) and 594 (red) as a secondary antibody (Molecular Probes, Eugene, OR). All obtained images were imported into Image-ProPlus, version 4.0 (Media Cybernetics, Sliver Spring, MD) for quantifying levels of GFAP-and SDF-positive staining.
Statistical Tests
All results were expressed as means ± standard deviation of the mean (SD). All experiments were done in triplicate. Data was evaluated statistically by ANOVA. Significance was considered to be less than 0.05. To account for any donor specific differences, all experiments were performed from at minimum of three donors.
RESULTS
Cell Specific Analysis of SDF-1 Expression
We first determined the expression pattern of SDF-1 in primary human cortical astrocytes, neurons, neural progenitor cells, and MDM by real-time RT-PCR. MDM (n = 2) and astrocytes (n = 3) (Ghorpade et al., 2003), neurons (n = 2) (Zheng et al., 1999), and neural progenitor cells (n = 3) (Peng et al., 2004) were analyzed for SDF-1 expression by real time RT-PCR. The results were standardized with GAPDH as an internal control. Astrocytes expressed the highest level of SDF-1 mRNA among all cell types. Neurons expressed 30–50% SDF-1 mRNA as compared to astrocytes. MDM and neural progenitor cells expressed extremely low levels of SDF-1 mRNA (MDM, 0.07%; NPC, 9.5% as compared to astrocytes) (see Fig. 1).
Fig. 1.
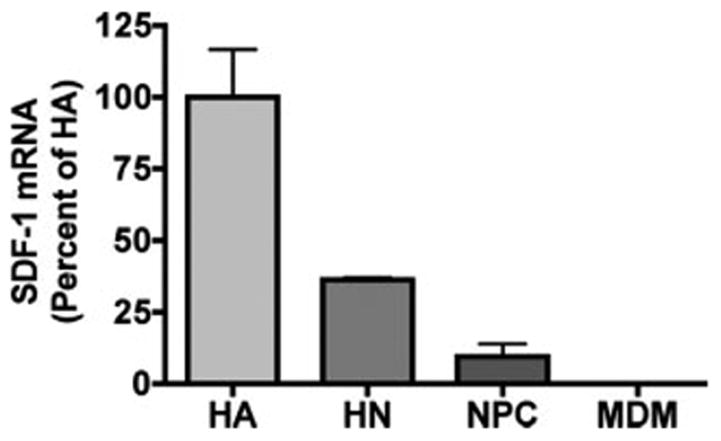
SDF-1 expression in astrocytes, neurons, neural progenitor cells, and MDM. Expression of SDF-1 in primary human cortical astrocytes (HA, n = 3), neurons (HN, n = 2), neural progenitor cells (NPC, n = 3), and MDM (n = 2) were assessed using real-time RT-PCR. SDF-1 mRNA expression was normalized to GAPDH as an internal gene expression control. Data is presented as a percentage of astrocyte expression, as means ± SD. Results represent average of two to three donors. Experiments were performed in triplicate.
MCM Induced SDF-1α Production by Astrocytes
Human astrocytes express the highest level of SDF-1 mRNA and represent the most abundant cell-type in the brain, we thus focused on astrocytes for SDF-1 production and regulation. Because MP are the principal cell type infected and a major source of neurotoxins in the brain, we reasoned that the interactions between MP and astrocytes would be a major mechanism for SDF-1 production in diseased brain. To test this hypothesis, MCM recovered from HIV-1-infected and immune-activated MDM were investigated for their ability to induce the production of SDF-1. Human astrocytes were treated with 25% MCM with/without HIV infection and/or LPS stimulation for 48 h. LPS-stimulated MCM induced a significant increase in SDF-1 production. Furthermore, HIV-1-infected and LPS-stimulated MCM induced significantly higher levels of SDF-1 production as compared to LPS-stimulated MCM alone (Fig. 2A). We also measured the SDF-1 in MCM and neither HIV-1-infected nor LPS-stimulated MCM showed detectable SDF-1 (data not shown), excluding the possibility that the measured SDF-1 originated from the MCM treatment.
Fig. 2.
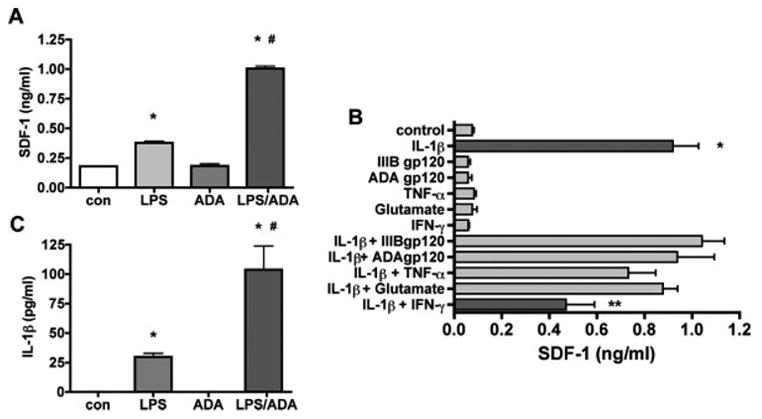
Conditioned media from HIV-1ADA infected or immune-activated macrophages induce SDF-1 production by astrocytes. A: Human astrocytes were treated with HIV-1-infected or LPS-stimulated MCM and the supernatants were assayed for SDF-1 production by Fluro ELISA at 48 h. B: Human astrocytes were treated with IL-1β (500 pg/mL), IFN-γ (100 ng/mL), TNF-α (50 ng/mL), and HIV-1 gp120 (1 nM) for 48 h. The supernatants were assayed for SDF-1 production by Fluro ELISA. C: Conditioned media from HIV-1ADA-infected or immune-activated macrophages IL-1β levels by ELISA, Data represent the mean ± SD of triplicate samples from a representative result of three experiments. * P < 0.001 in comparison to control. # P < 0.001 in comparison to LPS. ** P < 0.001 in comparison to IL-1β.
To assay the possible contributing factors to astrocyte SDF-1 production in MCM, we treated astrocytes with products of HIV-1-infected or immune-activated macrophage, including IL-1β, IFN-γ, TNF-α, and HIV-1 gp120 for 48 h. Among all tested factors, only IL-1β induced a significant increase in SDF-1 production. Additionally, IFN-γ-inhibited IL-1β induced SDF-1 production (Fig. 2B). To further extend these observations, a dose dependent response was determined for astrocytes treated with IL-1β (5, 50, 500, and 5000 pg/mL) for 24 h. IL-1β induced a dose dependent increase of SDF-1 production in astrocytes, the EC50 for this effect was 26.5 pg/ml (Fig. 3A). Astrocytes were also treated with 500 pg/mL IL-1β for 1, 2, 4, 8, 16, 24, and 48 h. The IL-1β-induced increase in SDF-1 mRNA expression was first noted at 8 h, and was most significantly observed at 24 h (Fig. 3B). The increase in SDF-1 protein was observed at both 24 and 48 h (Fig. 3C). IL-1β treatment did not significantly change the viability of astrocytes (data not shown), indicating that the increase in protein was not likely due to change in astrocyte viability.
Fig. 3.
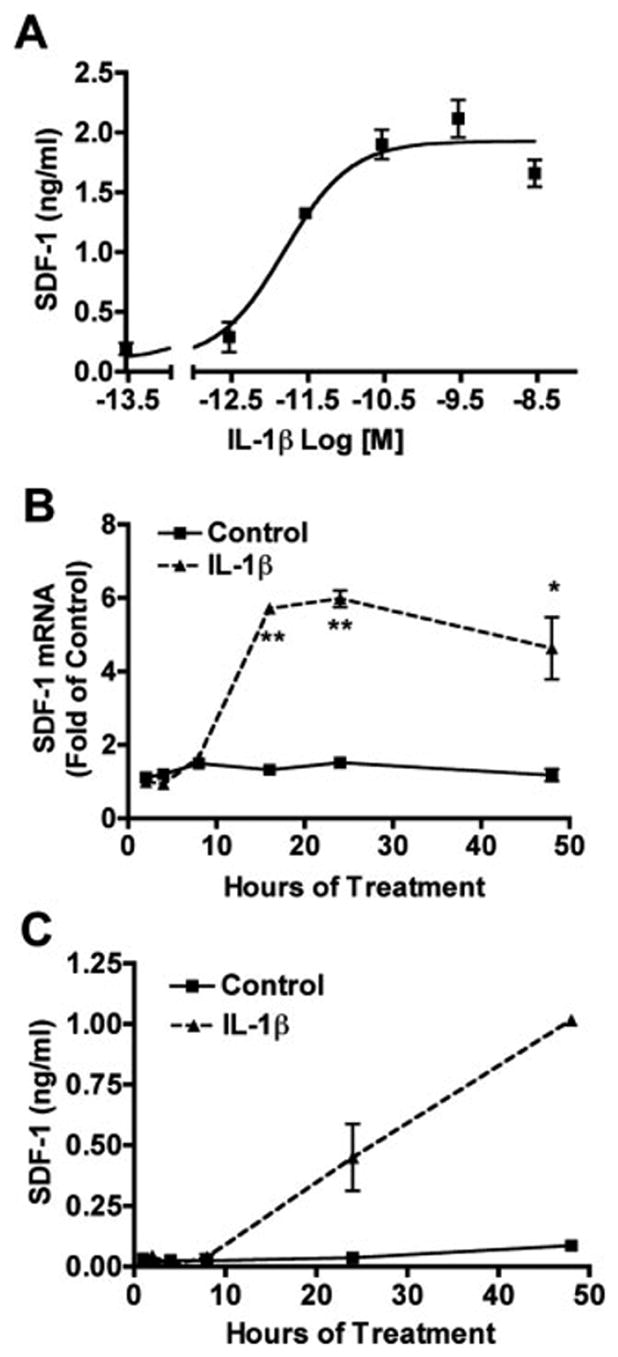
IL-1β induced SDF-1 production by human astrocytes. A: Astrocytes were treated with IL-1β (5, 50, 500, and 5,000 pg/mL) for 24 h and supernatants were assayed for SDF-1 by Fluro ELISA. B: Astrocytes were treated with 500 pg/mL IL-1β for 1, 2, 4, 8, 16, 24, and 48 h. mRNA expression of SDF-1 was quantitated by real time RT-PCR. SDF-1 mRNA expression was normalized to GAPDH as an internal gene expression control, presented as folds of control. C: Cell culture media was assayed for SDF-1 Fluro ELISA. Results are means ± SD from two independent experiments. * P < 0.05 in comparison to control. ** P < 0.001 in comparison to control.
IL-1β from HIV-1-Infected or Immune-Activated MDM Induced SDF-1 Production by Astrocytes
Gene expression of IL-1α and IL-1β can be induced very rapidly by activators of inflammation [e.g., bacterial products such as LPS, tumor necrosis factor (TNF-α), cellular injury, or hypoxia (Dinarello, 1998)]. To verify whether IL-1β is the major inducer of astrocyte SDF-1 production, IL-1β levels in MCM were measured by ELISA. LPS induced IL-1β production by MDM, whereas HIV-1-infection alone did not induce a significant increase in IL-1β production, but potentiated LPS stimulated IL-1β production (Fig. 2C). Further, SDF-1 production from astrocytes correlated with IL-1β concentration in MCM. We also tested macrophage-tropic strains BAL and JRFL (Zheng et al., 2001), and the same pattern of IL-1β production was observed as with the ADA strain. The data demonstrated an increase in SDF-1 production by human astrocytes exposed to immune-activated and HIV-1-infected MCM, and a correlation to IL-1β level (see Fig. 4).
Fig. 4.
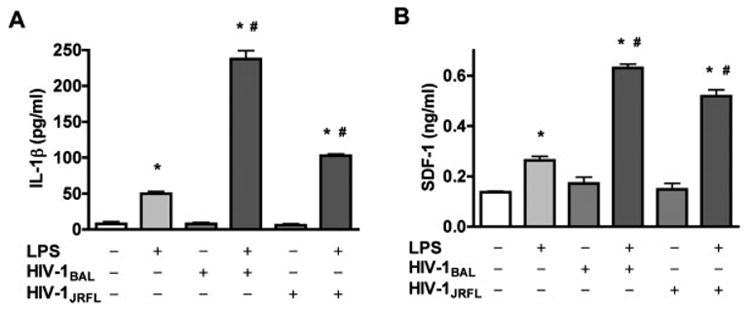
Conditioned media from HIV-1BAL- and HIV-1JRFL-infected or immune-activated macrophages induce astrocyte SDF-1α production. A: MDM were infected with other macrophage-tropic viral strains, HIV-1BAL and HIV-1JRFL. HIV-infected or immune-activated MCM were detected for IL-1β levels by ELISA. B: Human astrocytes were treated with HIV-infected or LPS-stimulated MCM for 48 h and the supernatants were assayed for SDF-1 production by Fluro ELISA. Data represent the mean ± SD of triplicate samples from a representative result of three experiments. * P < 0.001 in comparison to control. # P < 0.001 in comparison to LPS.
To validate whether MCM-induced SDF-1 production is through IL-1β, two approaches were used, IL-1Ra and IL-1β specific siRNA. IL-1Ra is a highly selective, competitive antagonist for IL-1, and appears to block all actions of IL-1, with no identified independent actions (O’Neill and Dinarello, 2000). To block the effect of IL-1β, astrocytes were pretreated with IL-1Ra (100 ng/mL) for 1 h and were then treated with 25% MCM. The data showed that IL-1Ra markedly reduces SDF-1 production (by 50% or more) induced by MCM at both the mRNA (data not shown) and protein levels (see Fig. 5), supporting IL-1β as a major mediator in MCM for the induction of SDF-1 from astrocytes.
Fig. 5.
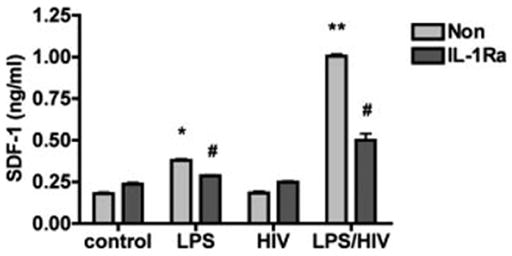
IL-1Ra abrogates MCM induced astrocyte SDF-1 production. Human astrocytes were pretreated with IL-1Ra (100 ng/mL) for 1 h and were then treated with 25% HIV-1ADA-infected or immune-activated MCM for 48 h. The supernatants were assayed for SDF-1 production by Fluro ELISA. Data represent the mean ± SD of triplicate samples from a representative result of three experiments. * P < 0.05 in comparison to control. ** P < 0.001 in comparison to control. # P < 0.05 in comparison to non IL-1Ra control.
To further confirm this observation, we used siRNA targeting IL-1β to block IL-1β production in MDM. To evaluate the transfection efficiency in primary macrophages, cells were first transfected with control siRNA siGLO (red fluorescence tagged siRNA) and analyzed by microscopy. Transfected (Figs. 6B,E, red) and total cells (Figs. 6A,D for phase; Figs. 6C,F for nuclear, blue) were counted after acquiring digital images. The transfection efficiency of siGLO reached 73–83% as measured 24 h post-transfection (Fig. 6G). HIV-infection induced no significant effect on siRNA transfection compared to non-infection cells (Control, 72.9% ± 6.4%; HIV, 82.6% ± 6.4%).
Fig. 6.
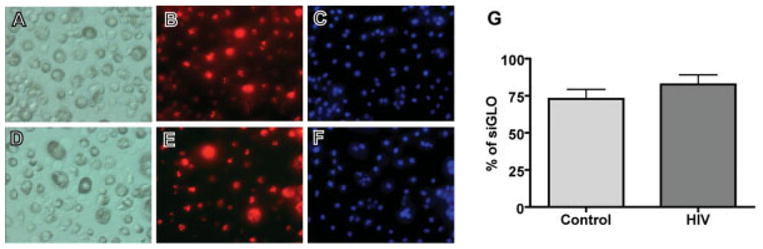
Control and HIV-1ADA-infected MDM were transfected with control siRNA siGLO (Dharmacon, Lafayette, CO). At 24 h post transfection, cells were incubated with Hoechst 33342 for nuclear staining. Transfected (red, B and E) and total cells (A and D for light phase;C andF for nuclear, blue) were counted after acquiring digital images (20 ×). A minimum of five fields was counted for each treatment condition; quantified data were shown (G). Results are means ± SD from two independent experiments.
MDM were then transfected with siRNA against IL-1β or non-specific control siRNA. Twenty-four hours post transfection, MDM were stimulated with LPS (0.1 μg/mL) for 3 h. MDM mRNA was collected at both 24 h post-transfection/3 h after stimulation and 48 h post-transfection/24 h after stimulation. MCM was collected 24 h after LPS stimulation (48 h post-transfection) for IL-1β ELISA. LPS induced a significant increase of IL-1β mRNA (510-fold at 3 h, 168-fold at 24 h compared to control), HIV-1 induced a minor increase (1.7-fold at 3 h, 2.1-fold at 24 h compared to control), and HIV-1 infection potentiated LPS induced increase of IL-1β mRNA expression at 3 h (609-fold as compared to control). The inhibitory effects of the targeted siRNA on IL-1β expression were observed at both 24 h post-transfection/3 h after stimulation and 48 h post-transfection/24 h after stimulation (Figs. 7A,B), while silMPORTER alone and control siRNA had no significant effect compared to control (data not shown). The production of IL-1β protein was also monitored by ELISA, revealing that the expression of IL-1β was markedly reduced 48 h post-transfection/24 h after stimulation (Fig. 7C).
Fig. 7.
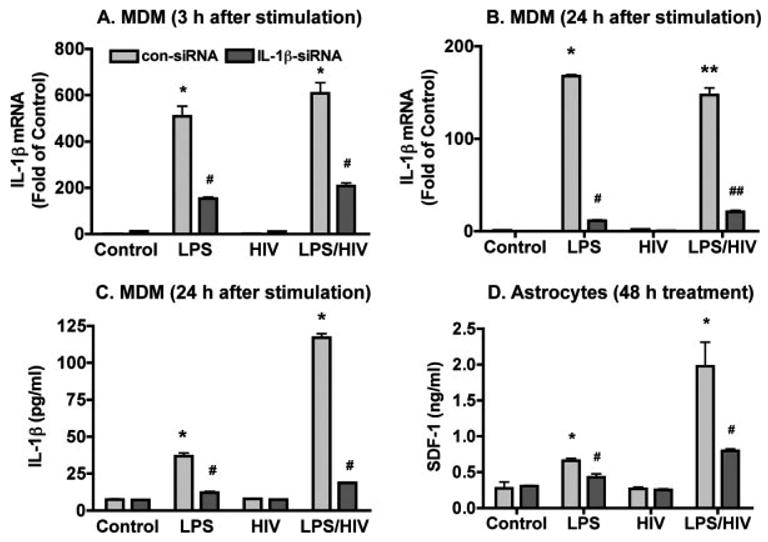
IL-1β siRNA abrogates MCM induced astrocyte SDF-1 production. A,B: Control and HIV-1ADA-infected MDM were transfected with siRNA targeting IL-1β or nonspecific control siRNA and then were stimulated with LPS (0.1 μg/mL) for 3 h. MDM mRNA was collected at both 24 h post-transfection/3 h after stimulation (A) and 48 h post-transfection/24 h after stimulation (B); IL-1β mRNA detection by real time RT-PCR. SDF-1 mRNA expression was normalized to GAPDH as an internal gene expression control, and data are presented as folds of control. C: MCM was collected 24 h after LPS stimulation (48 h post-transfection) for IL-1β ELISA. D: Human astrocytes were then stimulated by 25% MCM and astrocyte supernatants were collected for SDF-1 ELISA after 48 h stimulation. Data represent the mean ± SD of triplicate samples from a representative result of three experiments. * P < 0.05 in comparison to control. ** P < 0.001 in comparison to control. * P < 0.05 in comparison to nonspecific control siRNA (con-siRNA). ## P < 0.001 in comparison to con-siRNA.
Human astrocytes were then stimulated by 25% MCM and astrocyte supernatants were collected for SDF-1 ELISA 48 h after stimulation. SDF-1 production was prevented in astrocytes treated with IL-1β siRNA transfected MCM compared to control siRNA transfected MCM (Fig. 7D), suggesting that IL-1β, produced from HIV-1-infected and activated macrophages, is the major factor stimulating SDF-1 production in astrocytes.
SDF-1 is Upregulated in HIVE Mice
To investigate the role of macrophage in mediating SDF-1 production from astrocytes in vivo, we used a HIVE SCID mouse model of human disease. Human HZV-1ADA-infected MDM were intracranially injected into the basal ganglia of SCID mice. Histopathological changes observed in murine brain tissue parallels those seen in human HIVE as previously described (Dou et al., 2003; Persidsky et al., 1996). Seven days after injection, HIV-1-infected MDM were found in the putamen and cortex (Figs. 8G,H). We next identified and quantified SDF-1 distributed through the injected hemisphere by immunostaining with SDF-1 in serial 5 urn brain slices (Figs. 8B,E,I). Morphological changes in astrocytes were identified by immunostaining for GFAP antigen expression (see Fig. 8). GFAP and SDF-1 expression was quantified by determining the GFAP-positive or SDF-1-positive area as a percentage of the total image area per microscopy field and calculated for a 0.5 mm tissue area immediately surrounding the injection site.
Fig. 8.
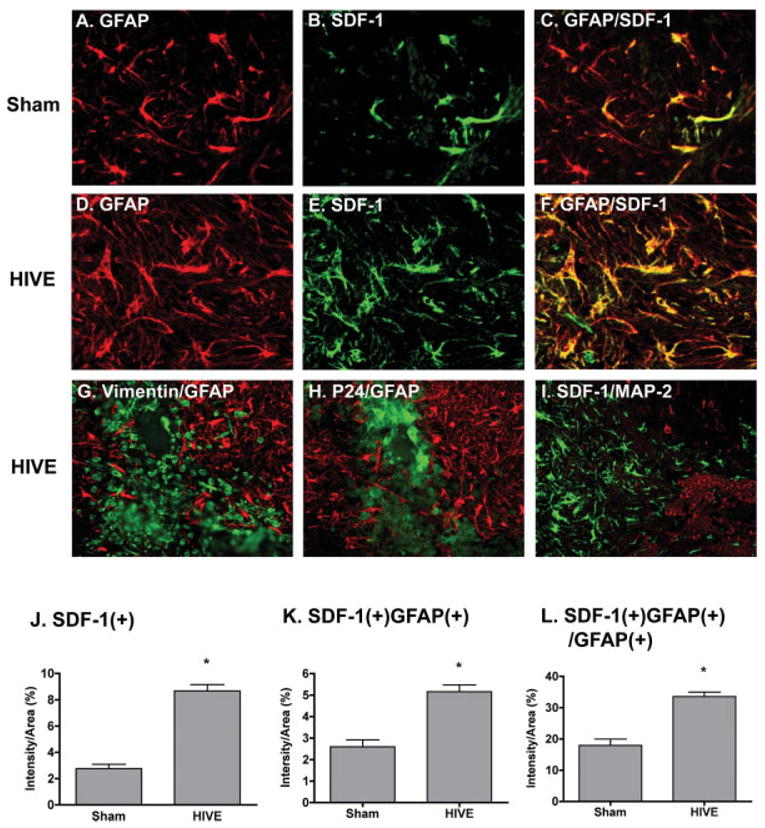
Immunohistological analysis of SDF-1 expression in HIVE mice. HIV-1ADA-infected MDM (5 × 105 cells in 5 μL) or media were intracranially injected into the basal ganglia of SCID mice (n = 4 per group). Mice were killed 7 days after injection and brain tissue was collected and subjected to immunocytochemical staining. A–F: Serial 5 μm paraformaldehyde-fixed paraffin-embedded coronal brain sections were immunolabeled with antibodies to GFAP (red) and SDF-1 (green) in both sham (A–C) and HIVE (D–F) group. G–I: Serial sections were stained with human vimentin (green) and GFAP (red) (G), HIV-1 p24 (green) and GFAP (red) (H), or MAP-2 (red) and SDF-1 (green) (I). Original magnification is 400 × for A–F; 200 × for G–I. J–L: GFAP and SDF-1 expression was quantified by determining the GFAP-positive or SDF-1-positive area as a percentage of the total image area per microscopy field and calculated for a window of tissue immediately surrounding the injection site. Data were shown as the percentage of SDF-positive area (J), the percentage of SDF and GFAP double-positive area (K), and the percentage of SDF and GFAP double-positive area in GFAP-positive area (L). Data represent the mean ± SEM. * P < 0.05 in comparison to sham.
Similar to previous observations, GFAP-positive astrocytes are hypertrophied with a reactive morphology in the injected hemisphere. SDF-1-positive cells were observed surrounding the injection site and co-localized with GFAP-positive cells (Figs. 8C,F). The percentage of SDF-positive area (intensity/area) was 8.68 ± 3.14 compared to 2.76 ± 1.9 on day 7 in HIVE versus sham-operated mice (Fig. 8J). The percentage of SDF and GFAP double-positive area (intensity/area) was 5.16 ± 2.09 compared to 2.60 ± 1.84 in HIVE versus sham-operated mice (Fig. 8K). Because some of the injected MDM stain for SDF-1, the ratio of double-stained SDF and GFAP to SDF-positive area is reduced in the HIVE SCID mice as opposed to sham. This may be due to the high expression of CXCR4 with potential SDF-1 binding on MDM. To evaluate the percentage of GFAP positive regions expressing SDF-1, SDF-1 and GFAP double positive areas were normalized by total GFAP positive area. The percentage of SDF-1 and GFAP double positive areas to total GFAP area was 33.55 ± 9.58 in HIVE samples compared to 17.96 ± 11.53 in sham-operated mice (Fig. 8L). This suggests that HIV-1-infected MDM induce more SDF-1 production in astrocytes.
A previous study has shown that patients with HIVE showed intense somato-dendritic neuronal SDF-1α immunoreactivity (Langford et al., 2002). To identify if neuronal cells also express SDF-1 in our HIVE model, we stained sections with MAP-2 and SDF-1. Few neurons survived around the injection site, but among remaining neurons, no SDF-1 co-localized with MAP-2 positive cells (Fig. 8I).
To correlate the expression of SDF-1 expression to IL-1β production, additional quantitative measurements of SDF and IL-1β mRNA were performed by real time RT-PCR. From ten mice, total RNA was extracted from 2 mm thick brain tissue sections from both the injection hemisphere (left hemisphere, LH) and the contralateral hemisphere (right hemisphere, RH). Mice inoculated with HIV-1-infected MDM (LH) showed a 3.5-fold increase in IL-1β expression in the injection hemisphere (LH) (from 1.10 to 4.59, P = 0.002, Fig. 9A) when compared to the contralateral hemisphere (RH). As a constitutively produced chemokine, basal SDF-1 expression in the brain is 250-fold as compared to the inducible cytokine IL-1β (data not shown). Despite its high basal levels, we observed a 12% increase in SDF-1 expression (from 1.02 to 1.14, P = 0.0249, Fig. 9B) in the injection hemisphere (LH) when compared to the contralateral hemisphere (RH). Furthermore, SDF-1 expression is significantly correlated with IL-1β expression (Fig. 9C). These data provide in vivo evidence that IL-1β is a major factor stimulating astrocyte SDF-1 production.
Fig. 9.
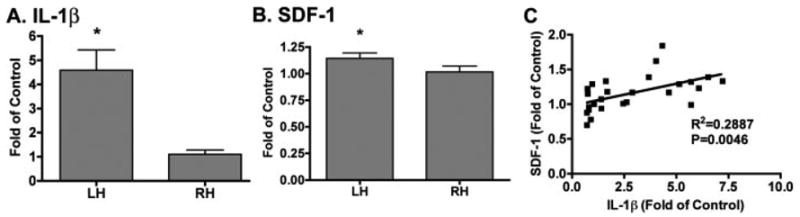
IL-1β and SDF-1 mRNA expression in HIVE mice. Ten mice were injected with HIV-1-infected MDM and RNA was extracted from 2 mm thick brain tissue of both the injection hemisphere (left hemisphere, LH) and the contralateral hemisphere (right hemisphere, RH) for real time RT-PCR. A,B: IL-1β (A) and SDF-1 (B) mRNA expression were normalized to GAPDH as an internal gene expression control and were presented as folds of RH. Data represent the mean ± SEM. * P < 0.05 in comparison to RH.C: Result express the correlation of SDF-1 mRNA expression to IL-1 mRNA expression.
DISCUSSION
In this report, we investigated the potential role of IL-1β in the stimulation of SDF-1 production by astrocytes. Immune-activated or HIV-1ADA-infected MDM conditioned media induces a significant increase in SDF-1 production by astrocytes. This effect was also observed in experiments with a panel of macrophage-tropic strains, including HTV-1BAL and HIV-1JRFL. Furthermore, we showed that astrocyte SDF-1 is dependent upon an increase of IL-1β in infected/activated MDM (Figs. 2 and 4). Recombinant human IL-1β is the best stimulator of SDF-1 from astrocytes in comparison to various macrophage produced cytokines and viral proteins such as TNF-α, IFN-γ, and HTV-1gp120 (Fig. 2B). Moreover, SDF-1 production in astrocytes mediated by MCM was prevented by IL-1Ra, a receptor antagonist of IL-1β (see Fig. 5). IL-1β siRNA was used to inhibit IL-1β production from macrophages, and also decreased MCM-mediated SDF-1 production in astrocytes (see Fig. 7). In a murine HIVE model, injection of HIV-1-infected MDM induced a significant increase of SDF-1 expression by astrocytes when compared to sham-operated control (see Fig. 8). Further, SDF-1 mRNA is increased in the injection site and is correlated with IL-1β mRNA expression (see Fig. 9). These observations provide evidence that IL-1β from HIV-1 infected and activated macrophages plays an essential role in astrocyte SDF-1 production.
Although patients with HIVE demonstrate intense somato-dendritic neuronal SDF-1α immunoreactivity (Langford et al., 2002), SDF-1 is primarily produced by astroglial cells within the CNS (Rostasy et al., 2003; Zheng et al., 1999). In the present study, we found that cultured human astrocytes expressed the most SDF-1 mRNA while cultured human neurons express 30–50% as compared to astrocytes (see Fig. 1), indicating that astrocytes are the major cell type for SDF-1 production. In vivo data also demonstrated that a majority of the SDF-1 positive cells around the injection site are GFAP-positive cells; neurons around the injection site were not found to express SDF-1 (Fig. 8I). Since there are few neurons remaining in the injection site, neurons as a source of SDF-1 during disease needs further investigation. Interestingly, macrophages, the principal target cells for HIV-1, expressed very low levels of SDF-1 (less than 1% as compared to astrocytes, Fig. 1). HIV-1 infection or activation does not significantly change the expression of SDF-1 in MDM (less than 5% compared to astrocytes, data not shown).
The importance of HIV-1-infected and immune-activated MP (macrophage and microglia) in HAD is widely recognized (Gendelman, 1997; Glass et al., 1995; Koenig et al., 1986; Nath and Geiger, 1998; Strizki et al., 1996; Wiley, 1995). MP contribute to the disease process by mediating brain inflammation through secretion of viral proteins or viral induction of cytokines that bind to glial cells and neurons (Merrill and Chen, 1991). Although MP are not the major SDF-1 producing cells, they may contribute to chemokine production through interactions with other cells, particularly astrocytes. Previous work demonstrated that SDF-1 mRNA is up-regulated in astrocytes treated with MCM (Zheng et al., 1999); however, the factors directly leading to SDF-1 production from astrocytes have not been identified. Except for IL-1β, none of the cellular and viral macrophage products were tested, including TNF-α, IFN-γ, HIV-1 gp120, or glutamate induced significant SDF-1 production in astrocytes (Fig. 2B). We observed that the MCM-mediated SDF-1 production in astrocytes correlated with an increase of IL-1β (Figs. 2 and 4). We tested IL-1β receptor antagonist IL-IRa and IL-1β siRNA (Figs. 5–7) and confirmed that IL-1β from HIV-1-infected and activated macrophage is the critical factor in up-regulating SDF-1 production in astrocytes.
IL-1 has been identified as a mediator of diverse forms of acute neurodegeneration and is also implicated in chronic neurological conditions (Gibson et al., 2004; Nesic et al., 2001; Rothwell et al., 1997). The IL-1 family currently is comprised of at least two known agonists, IL-1α and IL-1β. Elevated IL-1β has been demonstrated in CSF from patients infected with HIV-1 (Gallo et al., 1989). Infected macrophages stimulated with endotoxin generated readily detectable message for IL-1β as examined by Northern analysis (Locksley et al., 1988). Brain tissue from HIV-1-seropositive individuals and HAD patients also showed higher IL-1β RNA expression as compared to seronegative controls (Suryadevara et al., 2003). We demonstrated that HIV-1-infected MDM injection induced a significant increase of IL-1β mRNA in our in vivo study of murine HIVE model (see Fig. 9). HIV-1 induction of cytokines such as IL-1β may lead to an autocrine feedback loop involving further IL-1β production and induction of other cytokines and chemokines, including SDF-1α. The relation of SDF-1 production with IL-1β is confirmed by the significant correlation between IL-1β and SDF-1 mRNA in mouse brain (Fig. 9C).
The role of SDF-1α during the progression of HIVE is unclear. CXCR4, the receptor for SDF-1α, is a co-receptor for CXCR4-tropic HIV viruses (X4 viruses), and SDF-1, as a competitive inhibitor, prevents entry of X4 viruses (Amara et al., 1997; Oberlin et al., 1996). As a potent chemoattractant for leukocytes, it has been reported that SDF-1 induced transmigration of uninfected human lymphocytes and monocytes across the blood-brain-barrier (Wu et al., 2000). Numerous studies have suggested contrasting roles for SDF-1α in both promoting neuronal survival and triggering neuronal apoptosis in the brains of HIVE patients (Allen and Attwell, 2001; Bezzi et al., 2001; Kaul and Lipton, 1999; Meucci et al., 1998; Zhang et al., 2003; Zheng et al., 1999). SDF-1 may promote neuronal survival through activating antiapoptotic pathways (Khan et al., 2004; Meucci et al., 1998). However, high concentrations of SDF-1α may directly induce neuronal apoptosis though p38 mitogen-activated protein kinase (MAPK) via CXCR4 on neurons or indirectly through activated CXCR4 on astrocytes via TNF-α induced glutamate release (Allen and Attwell, 2001; Bezzi et al., 2001; Kaul and Lipton, 1999; Zhang et al., 2003; Zheng et al., 1999). SDF-1 may be cleaved by activated matrix metal-loproteinase-2 (MMP-2) to form the highly neurotoxic SDF-1 (5–67) (Zhang et al., 2003). SDF-1α may also induce astrocyte proliferation (Bajetto et al., 2001a). Recently, a study found that the activated and HIV-1-infected macrophages can indirectly induce astrocyte proliferation through up-regulation of SDF-1 and MMP production, implying a mechanism of astrogliosis in HAD (Okamoto et al., 2005). The up-regulation of SDF-1 via IL-1β produced by HIV-1-infected or immune-activated MDM support the potential role of SDF-1α in the development and progression of HIVE. The exact role and the mechanism by which astrocyte produced SDF-1 participates in this process requires further investigation.
Accumulating evidence indicates that endogenous neurogenesis may occur as part of an intrinsic brain self-repair process in neurodegenerative diseases. Studies have shown that chemokines are important for regulating the migration of neural progenitor cells (NPC) to sites of neuroinflammation (Belmadani et al., 2006; Imitola et al., 2004). SDF-1 mediated NPC migration in vitro has been demonstrated through CXCR4 mediated signaling pathways (Peng et al., 2004; Tran et al., 2004). SDF-1α has also been shown to be up-regulated and able to induce human neural stem/progenitor cell migratation in vivo toward an infarcted area (Imitola et al., 2004). In the present study, we find very low levels of SDF-1 mRNA expression by NPC (see Fig. 1) and up-regulation of this chemokine by astrocytes exposed to HIV-1-infected and immune-activated macrophages. Although the role of SDF-1α during the progression of HIVE is still unclear, the up-regulated expression of SDF-1α in astrocytes in response to HIV-1-infected and immune-activated macrophages may affect NPC function, eventually affecting neurogenesis and neuronal repair in HAD. In summary, since SDF-1 plays a role in multiple processes, including immune cell migration, neurotoxic activities, neuroregeneration, and astrogliosis, through interactions with blood leukocytes, neurons, neural progenitor cells, and astrocytes, SDF regulation is important in HAD pathogenesis.
Acknowledgments
We kindly acknowledge Li Wu, Emily Baily, and Shelley Herek who provided technical support for this work. Drs. Tsuneya Ikezu, Terry Hexum, and Myron Toews provided valuable comments and suggestions about the manuscript and the project. Julie Ditter, Robin Taylor, Myhanh Che, Na Ly, and Nell Ingraham provided outstanding administrative support.
Footnotes
Grant sponsor: National Institutes of Health; Grant numbers: R01 NS 41858, P20 RR15635, P01 NS043985, P01 NS31492.
References
- Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Attwell D. A chemokine-glutamate connection. Nat Neurosci. 2001;4:676–678. doi: 10.1038/89443. [DOI] [PubMed] [Google Scholar]
- Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizer J-L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent Internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Barbero S, Bonavia R, Piccioli P, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1α induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2001a;77:1226–1236. doi: 10.1046/j.1471-4159.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001b;22:147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Chao C, Hu S, Sheng W, Bu D-F, Bukrinsky M, Peterson P. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia. 1996;16:276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AID-GLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota M, Kleinschmidt A, Ceccherini-Silberstein F, Aloisi F, Mengozzi M, Mantovani A, Brack-Werner R, Poli G. Upregulated expression of interleukin-8, RANTES and chemokine receptors in human astrocytic cells infected with HIV-1. J Neurovirol. 2000;6:75–83. doi: 10.3109/13550280009006384. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16(56):457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- Dou H, Birusingh K, Faraci J, Gorantla S, Poluektova L, Maggirwar SB, Dewhurst S, Gelbard H, Gendelman HE. Neuroprotective activities of sodium valproate in a murine model of human immunodeficiency virus 1 encephalitis. J Neurosci. 2003;23:9162–9170. doi: 10.1523/JNEUROSCI.23-27-09162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen D, Lopez AL, Peng H, Niemann D, Williams C, Bauer M, Morgello S, Cotter RL, Ryan LA, Ghorpade A, Gendelman HE, Zheng J. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138(12):144–155. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- Gabuzda D, He J, Ohagen A, Vallat A. Chemokine receptors in HIV-1 infection of the central nervous system. Semin Immunol. 1998;10:203–213. doi: 10.1006/smim.1998.0133. [DOI] [PubMed] [Google Scholar]
- Gallo P, Frei K, Rordorf C, Lazdins J, Tavolato B, Fontana A. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989;23:109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Gendelman HE. The Neuropathogenesis of HIV-1-Dementia. In: Gendelman HE, Lipton SA, Epstein LG, Swindells S, editors. The neurology of AIDS. New York: Chapman and Hall; 1997. pp. 1–10. [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. Aids. 1997;11 (Suppl A):S35–S45. [PubMed] [Google Scholar]
- Ghorpade A, Holter S, Borgmann K, Persidsky R, Wu L. HIV-1 and IL-1β regulate Fas ligand expression in human astrocytes through the NF-κB pathway. J Neuroimmunol. 2003;141(12):141–9. doi: 10.1016/s0165-5728(03)00222-4. [DOI] [PubMed] [Google Scholar]
- Gibson RM, Rothwell NJ, Le Feuvre RA. CNS injury: the role of the cytokine IL-1. Vet J. 2004;168:230–237. doi: 10.1016/j.tvjl.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385(6617):645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8(10):595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefcoat AM, Hotchkiss JA, Gerber V, Harkema JR, Basbaum CB, Robinson NE. Persistent mucin glycoprotein alterations in equine recurrent airway obstruction. Am J Physiol Lung Cell Mol Physiol. 2001;281:L704–L712. doi: 10.1152/ajplung.2001.281.3.L704. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MZ, Brandimarti R, Patel JP, Huynh N, Wang J, Huang Z, Fatatis A, Meucci O. Apoptotic and antiapoptotic effects of CXCR4: is it a matter of intrinsic efficacy? Implications for HIV neuropathogenesis. AIDS Res Hum Retroviruses. 2004;20:1063–1071. doi: 10.1089/aid.2004.20.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Canto MCD, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kolson DL, Gonzalez-Scarano F. HIV and HIV dementia. J Clin Invest. 2000;106:11–13. doi: 10.1172/JCI10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Sanders VJ, Mallory M, Kaul M, Masliah E. Expression of stromal cell-derived factor 1α protein in HIV encephalitis. J Neuroimmunol. 2002;127(12):115–126. doi: 10.1016/s0165-5728(02)00068-1. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the α-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Dementia associated with the acquired immunodeficiency syndrome. New Engl J Med. 1995;16:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Crowe S, Sadick MD, Heinzel FP, Gardner KD, Jr, McGrath MS, Mills J. Release of interleukin 1 inhibitory activity (contra-IL-1) by human monocyte-derived macrophages infected with human immunodeficiency virus in vitro and in vivo. J Clin Invest. 1988;82:2097–2105. doi: 10.1172/JCI113831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4 and SDF 1 deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan A, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Chen IS. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991;5:2391–7. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex. II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma. 2001;18:947–956. doi: 10.1089/089771501750451857. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000;21(5):206–209. doi: 10.1016/s0167-5699(00)01611-x. [DOI] [PubMed] [Google Scholar]
- Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Wang X, Baba M. HIV-1-infected macrophages induce astrogliosis by SDF-1α and matrix metalloproteinases. Biochem Biophys Res Commun. 2005;336:1214–1220. doi: 10.1016/j.bbrc.2005.08.251. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1 mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [see comments] [PMC free article] [PubMed] [Google Scholar]
- Reiss K, Mentlein R, Sievers J, Hartmann D. Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience. 2002;115:295–305. doi: 10.1016/s0306-4522(02)00307-x. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, Hunter DD, Nath A, Hedreen JC, Navia BA. SDF-1α is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62:617–626. doi: 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest. 1997;100:2648–2652. doi: 10.1172/JCI119808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TW, AP IJ. Principles of agonism: undressing efficacy. Trends Pharmacol Sci. 1998;19(11):433–436. doi: 10.1016/s0165-6147(98)01262-0. [DOI] [PubMed] [Google Scholar]
- Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- Strizki JM, Albright AV, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadevara R, Holter S, Borgmann K, Persidsky R, Labenz-Zink C, Persidsky Y, Gendelman HE, Wu L, Ghorpade A. Regulation of tissue inhibitor of metalloproteinase-1 by astrocytes: links to HIV-1 dementia. Glia. 2003;44:47–56. doi: 10.1002/glia.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham TN, Lazarini F, Franceschini IA, Lachapelle F, Amara A, Dubois-Dalcq M. Developmental pattern of expression of the alpha chemokine stromal cell-derived factor 1 in the rat central nervous system. Eur J Neurosci. 2001;13:845–856. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during acquired immune deficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA. Quantitative neuropathologic assessment of HIV-1 encephalitis. Curr Top Microbiol Immunol. 1995;202:55–61. doi: 10.1007/978-3-642-79657-9_4. [DOI] [PubMed] [Google Scholar]
- Wu DT, Woodman SE, Weiss JM, McManus CM, D’Aversa TG, Hesselgesser J, Major EO, Nath A, Berman JW. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol. 2000;6(Suppl 1):S82–S85. [PubMed] [Google Scholar]
- Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM, Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin M, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng Y, Gelbard H, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Persidsky Y, Williams CE, Cotter RL, Zink W, Ryan L, Ghorpade A, Lewis K, Gendelman HE. HIV-1 infected immune competent mononuclear phagocytes influence the pathways to neuronal demise. Neurotoxi Res. 2001;3:461–484. doi: 10.1007/BF03033204. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]


