Abstract
A recombinant Eimeria protozoan protein antigen (rEA) has been shown to have antitumor and antiviral activity. The purpose of this study was to determine the effect of rEA treatment alone or in combination with an agonist cocktail consisting of granulocyte macrophage colony stimulating factor (GM-CSF), interferon gamma (IFN-γ), interleukin 4 (IL-4), and anti CD-40 antibody, in the treatment of Banzi virus (BV) disease in BALB/c mice. Treatment with rEA resulted in a significant increase in survival, weight gain, and mean day to death in BV-infected mice and resulted in a significant decrease in brain virus titer. Treatment with rEA, in combination with a 4-agonist cocktail, improved disease parameters to a greater degree than rEA treatment alone. The effect of treatment with a reduced concentration of agonist cocktail or fewer components of the agonist cocktail, in combination with rEA, on disease outcome in BV-infected mice was also investigated. Treatment with rEA, alone or in combination with agonist cocktail, 24 hours after virus challenge did not improve disease. Treatment with rEA, alone or in combination with an agonist cocktail, is efficacious for the prophylaxis of BV infection in mice.
Keywords: Banzi virus, Eimeria, cytokine, treatment, mice, rEA, Barrogen, immune modulation
1. Introduction
A protein was recently isolated from a protozoan, Eimeria spp., that modulates innate immunity through the upregulation of inflammatory modulators, including interleukin (IL)-12, monocyte chemoattractant protein (MCP)-1, IL-6, tumor necrosis factor (TNF)-alpha, and interferon (IFN)-gamma (Rosenberg et al., 2005). This Eimeria antigen (EA) has been shown to have anticancer activity in mice (Rosenberg et al., 2005). Prophylactic treatment with a recombinant version of the Eimeria antigen (rEA) significantly improved disease in mice infected with Punta Toro virus (Gowen et al., 2005) and is currently in Phase I clinical trials for cancer in humans (Rosenberg, personal communication). Synergistic efficacy of rEA and an agonist cocktail, consisting of GMCSF, IFN-γ, IL-4, and anti-CD-40 antibody, has also been observed in in vitro and in vivo studies in a cancer model (Rosenberg et al., 2005). All possible agonist cocktail combinations were tested in vivo by injecting mice with the cocktail and measuring the amount of IL-12 induced at 6 hrs post injection by ELISA (Rosenberg et al., 2005). The cocktails selected for use in this study induced the largest amounts of IL-12, typically 2-10x that induced by rEA alone (Rosenberg, unpublished data).
Banzi virus (BV) is a flavivirus belonging to the Uganda S serocomplex and is closely related to yellow fever virus, an NIAID Category C pathogen (Fulop, Barrett, and Titball, 1995). BV causes periodic cases of febrile illness in humans, and the infection in mice, characterized by encephalitis and high mortality, is used as a model for flavivirus-induced encephalitis in the laboratory (Jacoby and Bhatt, 1976; Smee et al., 1995).
Potential therepeutics for the treatment of flavivirus infections in experimental models include immune modulators including interferons, ribavirin, synthetic RNAs, and others (Leyssen et al., 2003; Morrey et al., 2004; Pantelic, Sivakumaran, and Urosevic, 2005; Pinto et al., 1990; Pinto, Morahan, and Brinton, 1988; Singh et al., 1989; Smee et al., 1991). Immune modulators generally have broad antiviral efficacy, but generally are efficacious only with prophylactic treatment. The purpose of this study was to determine if the immunomodulator rEA, alone or in combination with various agonists, would be effective in treating BV disease in experimentally infected mice.
2. Materials and Methods
2.1. Banzi virus
The H336 strain of BV was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and used after 3 passages in vero cells and titrated in mice prior to this study. A 10-3 dilution (103 cell culture 50% infectious doses [CCID50] or 20 50% lethal doses) of the virus was prepared, and animals were injected intraperitoneally (i.p.) with 0.1 ml of the diluted virus.
2.2. Animals
Female BALB/c mice with ages of 5-6 weeks obtained from Charles River Laboratories (Wilmington, MA) were used. Animals were randomly assigned to cages and individually marked with eartags. All were fed standard mouse chow and tap water ad libitum.
2.3. Facilities
Although BV is categorized as a BSL-2 pathogen (Richmond and McKinney, 1999), to assure personnel safety, experiments were conducted in the BSL-3 animal suite at Utah State University Laboratory Animal Research Center (LARC). Standard operating procedures for BSL-3 were used.
2.4. Test Article
All rEA and rEA + agonist preparations, along with the respective placebo controls, were supplied by Barros Research Institute (Holt, MI) as aqueous solutions ready for injection. The rEA was used at 1.0 or 0.1 μg/mouse/treatment (50 or 5 μg/kg). The 4-agonist cocktail consisted of granulocyte macrophage colony stimulating factor (GMCSF), interferon gamma (IFN-γ), interleukin-4 (IL-4), and anti-CD-40 antibody (Ab) at concentrations of 1 μg/kg, 3 μg/kg, 1 μg/kg and 50 μg/kg, respectively. The 3-agonist cocktail was as above without anti-CD-40 antibody and 2-agonist consisted of GM-CSF and IFN-γ. All rEA preparations were stored at 4 °C until use. The rEA and rEA + agonist preparations were prepared so that an i.p. injection of 0.4 ml/mouse yielded the desired concentration. The rEA diluent consisted of 0.1% bovine serum albumin in phosphate buffered saline. Ampligen was provided by HEMISPHERx (Philadelphis, PA) as a viscous 2.4 mg/ml solution and used as a positive control in these studies.
2.5. Brain virus titration
Brain samples from 5 mice from each group were homogenized in 1 ml MEM 2% FBS. Ten-fold dilutions were made of the homogenate, which were then plated on Vero-76 cells, incubated for 6 days, and examined for presence or absence of cytopathic effect (CPE) as indication of infectious virus.
2.6. Experimental design
In an initial dose response experiment, mice were treated i.p. 4 hrs prior to and 2 days post-virus inoculation (dpi) with 10, 1, or 0.1 μg of rEA to determine optimal dose. Brain samples were taken from 5 mice in each group for BV titration by infectious cell culture assay as described in section 2.5. In a following experiment, mice were treated i.p. with 1.0 or 0.1 rEA or rEA + 4-agonist following the same treatment schedule. A third experiment evaluated post-infection efficacy of rEA + 4-agonist administered 1 and 3 dpi. Other experiments were conducted to determine the optimal agonist cocktail components and concentrations. Mice were treated with 12 mg/kg ampligen −4 h and 2 dpi as a positive control in each experiment. Sham-infected control mice were treated with rEA, rEA + agonist, or placebo as toxicity controls. Percent survival and mean weight change were calculated based on the number of animals still alive 21 dpi and the weight difference between 0 and 8 dpi.
2.7. Statistical analysis
Survival data were analyzed using Wilcoxon log-rank survival analysis (JMP Software, SAS Institute Inc.,). Weight change was analyzed using Students t-test.
3. Results
3.1. rEA Treatment
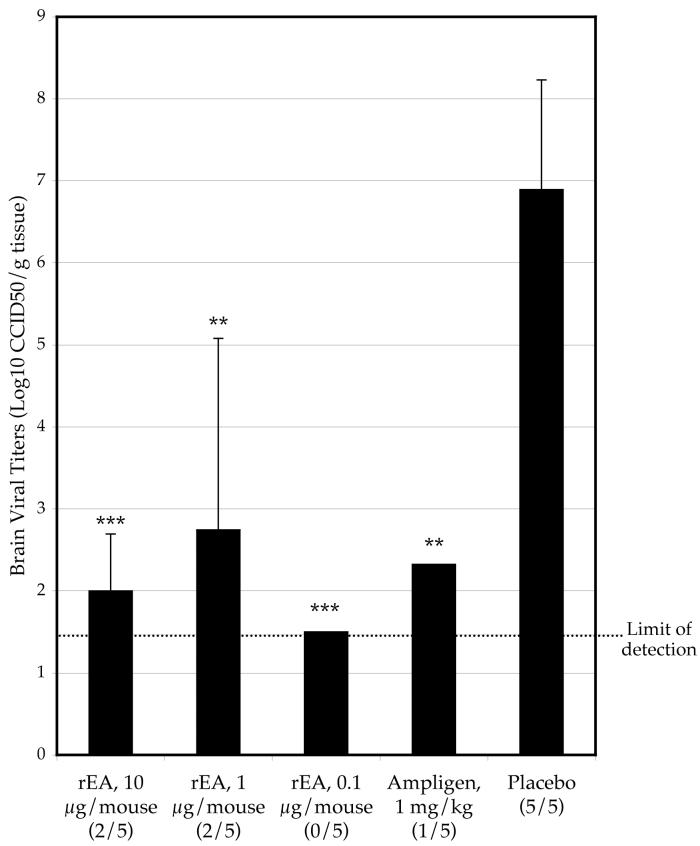
Mice treated −4 h and 2 dpi with rEA at 1.0 or 0.1 μg/mouse had a significantly higher survival rate than mice treated with placebo, whereas 10 μg/mouse did not significantly improve survival (Table 1). Brain virus titers were also reduced in mice treated with rEA (Fig 1). Weight change was improved with 10 or 1 μg/mouse rEA treatment as compared with placebo (Table 1). Mean day to death was not effected in the initial experiment evaluating rEA, but a subsequent experiment showed a significant improvement in this parameter with rEA treatment at 1 or 0.1 μg/mouse. No toxicity was apparent in any rEA treatment groups as indicated by weight loss or mortality. Ampligen treatment, given at the same treatment schedule, resulted in higher survival than rEA treatment in all of the experiments (Table 1-4).
Table 1.
The effect of various concentrations of rEA, or ampligen, administered i.p. −4 h and 2 dpi, on survival, mean day to death, and weight change in mice infected with Banzi virus.
| Toxicity controls | Infected, treated | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Regimen | Dosage | Alive /total |
Wt. changea | Alive / total |
MDDb | Wt. change |
| rEA | −4 h, 2 dpi | 10 μg | 3/3 | 0.6 | 4/10 | 14.2 ± 3.5 | −0.2 |
| rEA | −4 h, 2 dpi | 1 μg | 3/3 | 0.2 | 7/10*** | 14.3 ± 1.5 | −0.1 |
| rEA | −4 h, 2 dpi | 0.1 μg | 3/3 | 0.7 | 5/10* | 8.4 ± 5.5 | −3.6 |
| Ampligen | −4 h, 2 dpi | 1 mg/kg | 3/3 | -- | 10/10*** | >21 ± 0.0*** | 0.4 |
| Placebo | −4 h, 2 dpi | -- | 3/3 | 1.2 | 2/20 | 11.4 ± 2.9 | −2.7 |
difference between initial weight and weight on 8 days post-virus injection.
mean day to death of mice dying prior to 21 dpi.
P ≤ 0.001,
P ≤ 0.05, as compared with placebo.
Figure 1.
Effect of various concentrations of rEA on the reduction of day 8 Banzi virus titers in the brains of infected mice (# with detectable titer/total tested) (***P<0.001, **P<0.01, as compared with placebo).
Table 4.
Effect of concentration reduction of 4-agonist in combination with rEA treatment on survival, mean day to death, and weight change in mice infected with Banzi virus. 3-agonist at standard concentration or ampligen were also used to treat Banzi-infected mice.
| Toxicity controls | Infected, treated | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Dosage | Treatment Schedule | Alive /total |
Wt. changea | Alive /total |
MDDb | Wt. change |
| rEA + 4-ag [1/3]c | 0.1 μg | −4 h, 2 dpi, i.p. | 3/3 | 0.8 | 8/10 | 13.0 ± 0.0** | 0.0 |
| rEA + 4-ag [1/10] | 0.1 μg | −4 h, 2 dpi, i.p. | -- | -- | 2/10 | 11.8 ± 1.8 | 0.0 |
| rEA + 3-ag | 0.1 μg | −4 h, 2 dpi, i.p. | -- | -- | 8/10 | 11.0 ± 0.0** | 0.3 |
| Ampligen | 12 mpk | −4 h, 2 dpi, i.p. | -- | -- | 10/10*** | ≥21 ± 0.0*** | 0.7 |
| Placebo + 4-ag [1/3] | -- | −4 h, 2 dpi, i.p. | 3/3 | 1.2 | 6/10* | 10.3 ± 2.1* | −0.6 |
| Placebo + 4-ag [1/10] | -- | −4 h, 2 dpi, i.p. | -- | -- | 4/10 | 10.0 ± 1.5 | −1.2 |
| Placebo + 3-ag | -- | −4 h, 2 dpi, i.p. | -- | -- | 5/10* | 11.4 ± 1.7* | −0.1 |
| Placebo | -- | −4 h, 2 dpi, i.p. | 3/3 | 1.3 | 2/10 | 9.0 ± 1.3 | −1.7 |
| Normal Controls | -- | -- | 3/3 | 1.2 | -- | -- | -- |
difference between initial weight and weight on 9 days post-virus injection.
mean day to death of mice dying prior to 21 dpi.
Brackets indicate concentration reduction, eg [1/3] is 1/3 of the standard agonist cocktail concentration.
P ≤ 0.001,
P ≤ 0.01,
P ≤ 0.05, as compared with respective placebo (i.e. Placebo + 3-agonist is the placebo control group for rEA + 3-agonist).
3.2. Combination rEA + agonist treatment
Combination treatment of rEA (1.0 or 0.1 μg) with a 4-agonist cocktail (GM-CSF, IFN-γ, IL-4, and anti-CD-40 Ab) resulted in a significant improvement in survival as compared with placebo in combination with 4-agonist cocktail (Table 2). There was a significant difference (P<0.05) in rEA (0.1 μg) + 4-agonist as compared with rEA (0.1 μg) treatment alone. There was not a significant difference in placebo + 4-agonist treatment as compared with placebo alone, although more mice survived in the placebo + 4-agonist treatment group (Table 2). A second study testing rEA + 4-agonist treatment administered as above, also resulted in significantly improved survival (Table 3). There was no significant extension in mean day to death as compared with placebo + 4-agonist, although there was a trend towards improvement. Post-virus exposure treatment with rEA did not improve survival in mice infected with BV (Table 3).
Table 2.
Effect of rEA, rEA + 4-agonist, or ampligen, administered i.p. −4 h and 2 dpi, on disease parameters in mice infected with Banzi virus.
| Toxicity controls | Infected, treated | |||||
|---|---|---|---|---|---|---|
| Treatment | Dosage | Alive /total |
Wt. changea | Alive / total |
MDDb | Wt. change |
| rEA | 1 μg | 3/3 | 0.4 | 7/15** | 13.0 ± 1.6** | −1.0 |
| 0.1 μg | 3/3 | -- | 7/15** | 13.1 ± 1.7** | −1.4 | |
| rEA + 4-ag | 1 μg | 3/3 | −0.3 | 10/15*** | 15.2 ± 1.1*** | 0.5 |
| 0.1 μg | 3/3 | -- | 13/15*** | 12.0 ± 1.4** | −0.3 | |
| Ampligen | 12 mpg | 3/3 | -- | 9/10 | 15.0 ± 0.0*** | −0.1 |
| Placebo +4-ag | -- | 3/3 | 1.0 | 3/15 | 10.1 ± 2.2 | −3.5 |
| Placebo | -- | 3/3 | 0.7 | 0/15 | 10.5 ± 1.2 | −3.5 |
difference between initial weight and weight on 8 days post-virus injection.
mean day to death of mice dying prior to 21 dpi.
P ≤ 0.001,
P ≤ 0.01, as compared with respective placebo (i.e. Placebo + 4-agonist is the placebo control group for rEA + 4-agonist).
Table 3.
Effect of pre- and post-virus challenge treatment with rEA + 4-agonist, pretreatment with 2-agonist, or ampligen treatment on disease parameters in Banzi virus-infected mice.
| Toxicity controls | Infected, treated | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Regimen | Dosage | Alive /total |
Wt. changea | Alive / total |
MDDb | Wt. change |
| rEA + 4-agonist | −4 h, 2 dpi | 0.1 μg | 3/3 | 0.5 | 8/10** | 10.5 ± 0.7 | −1.0 |
| rEA + 4-agonist | 1, 3 dpi | 0.1 μg | -- | -- | 0/10 | 11.3 ± 1.9 | −3.1 |
| rEA + 2-agonist | −4 h, 2 dpi | 0.1 μg | 3/3 | −0.1 | 6/10** | 15.0 ± 2.0** | 0.0 |
| Ampligen | −4 h, 2 dpi | 12 mpk | -- | -- | 8/8*** | >21 ± 0.0*** | 0.2 |
| Placebo +4-agonist | −4 h, 2 dpi | -- | -- | -- | 3/10 | 12.7 ± 1.6 | −0.9 |
| Placebo + 2-agonist | −4 h, 2 dpi | -- | -- | -- | 2/10 | 11.0 ± 1.5 | −1.7 |
| Placebo | −4 h, 2 dpi | -- | 3/3 | 1.4 | 1/10 | 9.8 ± 0.8 | −3.0 |
difference between initial weight and weight on 8 days post-virus injection.
mean day to death of mice dying prior to 21 dpi.
P ≤ 0.001,
P ≤ 0.01, as compared with respective placebo (i.e. Placebo + 4-agonist is the placebo control group for rEA + 4-agonist).
Reduction of the agonist cocktail to a 3-agonist formulation of GM-CSF, IFN-γ, and IL-4 provided similar levels of protection to that of the 4-agoinst cocktail, when combined with rEA (Table 4). Further reduction in the composition of the agonist cocktail to GM-CSF and IFN-γ in combination with rEA treatment significantly improved survival of BV-infected mice, although survival was slightly diminished as compared with rEA + 4 agonist treatment (Table 3).
3.3. Dose-response effects of agonist
Combination treatment of rEA (0.1 μg/treatment) + 4-agonist at 1/3 of the standard agonist concentration (4-agonist [1/3]) was effective in improving survival and extending the mean day to death as compared with placebo only, but was not significantly different from placebo + 4-agonist [1/3] (Table 4), although this treatment significantly improved survival as compared with placebo alone (P<0.001). Treatment with placebo + 4-agonist [1/3] or treatment with placebo + 3-agonist cocktail resulted in a slightly significant improvement in survival as compared with placebo only (Table 4). The lower dilution [1/10] of 4-agonist cocktail had no significant effect on improvement of survival, mean day to death, or weight change (Table 4). The endpoint of efficacy for 4-agonist cocktail concentration is between [1/3] and [1/10], when combined with rEA treatment.
4. Discussion
The rEA protein, isolated from Eimeria sp., was effective in treating BV infection when administered 4 h prior to viral challenge. Treatment with rEA lessened weight loss, inhibited virus replication or reduced virus titers in the brain, and prolonged mean day to death, although results seem variable. It appears that the 10 μg/mouse dose was ineffective, as survival in mice treated with this dosage was lower than survival in groups of mice treated with 1 or 0.1 μg/mouse, and survival in this group was not significantly different than survival of placebo-treated mice. The dose/response curve for rEA treatment both in vitro and in vivo for cancer shows a bell-shaped resonse curve, indicating that 10 μg/mouse may exceed the optimum dose for treatment of the BV (Rosenberg, unpublished data). No direct toxicity was observed in the sham-infected, control animals at any dose tested.
Treatment with rEA elicits the production of IL-12 as well as other inflammatory cytokines and has been shown to have anti-tumor properties in mice (Rosenberg et al., 2005). The components of the agonist cocktails were chosen on the basis of maximizing IL-12 induction both in vitro and in vivo, as well as their ability to enhance the activity of rEA in the cancer model (Rosenberg, unpublished data). Production of IL-12 can result in many downstream events including induction of IFN-γ production by NK cells (Ellermann-Eriksen, 2005) and downregulation of the Th2 response (Rollier et al., 2005). IL-12 may be helpful in clearance of viral infection, but has been shown to not be necessary for the resolution of vesicular stomatitis virus and murine cytomegalovirus infections (Dix and Cousins, 2004; Ireland, Palian, and Reiss, 2005).
Combination treatment of rEA with an agonist cocktail improved survival, lessened weight change, and prolonged mean day to death to a greater extent than monotherapy with rEA. Co-treatment of rEA with other inflammatory cytokines may allow for a greater stimulation of an early immune response, which has been shown to be important for clearance of West Nile virus infection in mice (Diamond et al., 2003). Components of the agonist cocktail have been shown to have activity when given alone. Treatment with IFN-γ has been shown to reduce the number of dengue-positive cells in vitro (Diamond et al., 2000). GM-CSF, IFN-γ, and IL-4 have been shown to be important components of antiviral response to different flavivirus infections (Chaturvedi et al., 1999; Cheeran et al., 2005; Hasegawa, Satake, and Kobayashi, 1990; Quaresma et al., 2006). Thus exogenous treatment with these cytokines may be responsible for the resolution of BV infection observed in this study.
Removal of anti-CD-40 antibody from the agonist cocktail did not result in a diminished antiviral effect, suggesting that the anti-CD-40 antibody is not required to enhance the efficacy of rEA in this viral model. The reduction of agonist cocktail to GMCSF and IFN-γ in combination with rEA improved survival significantly when compared with placebo + 2-agonist-treated animals, although this improvement was not significantly different from rEA alone. It appears that rEA alone protects around 50% of the mice, which is comparable to 60% protection in groups of mice treated with rEA + 2-agonist cocktail. The addition of IL-4 to the 2-agonist formulation improved survival to around 80%. IL-4 reduces induction of IL-12 as compared with rEA in simple (i.e. rEA + IL-4) as well as some binary mixtures (e.g. rEA + IL-4 + anti-CD-40), however, inclusion of IL-4 in the 3- and 4-agonist cocktails showed an enhanced effect in inducing IL-12 over cocktails without IL-4 (Rosenberg, unpublished data).
Treatment with rEA + 4-agonist, administered 24 h after BV challenge, did not improve disease. It was not unexpected that post-virus exposure treatment with rEA would not be effective in improving disease outcome. Ampligen (polyI:polyC12U), another immunomodulatory compound, given after BV challenge results in diminished results (unpublished data), which is similar to published data involving other flaviviral encephalitides (Leyssen et al., 2003; Morrey et al., 2004). However, treatment of mice with rEA without agonists, administered 36 h after challenge with Punta Toro virus, a member of the Bunyaviridae family, resulted in significant protection, as measured by increased survival and decreased serum virus (Gowen et al., 2005). The lack of efficacy with post-virus exposure treatment may be due to differences in infection dynamics or virulence of BV as compared with PTV. Although rEA was not effective therapeutically against BV infection, this compound may be very useful in infections with nonencephalitic viruses, as demonstrated by effective post-infection treatment of Punta Toro, or in cancer treatment.
Acknowledgements
We thank Maysun Ali and Luci Wandersee for their work in animal treatments and data recording. This work was supported by Contract NO1-AI-15435 from the Virology Branch, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Justin G. Julander, Institute for Antiviral Research, Utah State University, Logan, UT..
John W. Judge, Barros Research Institute, Holt, MI.
Aaron L. Olsen, Institute for Antiviral Research, Utah State University..
Barnett Rosenberg, Barros Research Institute..
Kristiina Schafer, Institute for Antiviral Research, Utah State University..
Robert W. Sidwell, Institute for Antiviral Research, Utah State University..
References
- Chaturvedi UC, Elbishbishi EA, Agarwal R, Raghupathy R, Nagar R, Tandon R, Pacsa AS, Younis OI, Azizieh F. Sequential production of cytokines by dengue virus-infected human peripheral blood leukocyte cultures. J Med Virol. 1999;59(3):335–40. doi: 10.1002/(sici)1096-9071(199911)59:3<335::aid-jmv13>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cheeran MC, Hu S, Sheng WS, Rashid A, Peterson PK, Lokensgard JR. Differential responses of human brain cells to West Nile virus infection. J Neurovirol. 2005;11(6):512–24. doi: 10.1080/13550280500384982. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E. Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol. 2000;74(11):4957–66. doi: 10.1128/jvi.74.11.4957-4966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198(12):1853–62. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix RD, Cousins SW. Interleukin-2 immunotherapy and AIDS-related cytomegalovirus retinitis. Curr HIV Res. 2004;2(4):333–42. doi: 10.2174/1570162043351066. [DOI] [PubMed] [Google Scholar]
- Ellermann-Eriksen S. Macrophages and cytokines in the early defence against herpes simplex virus. Virol J. 2005;2(1):59. doi: 10.1186/1743-422X-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop LD, Barrett AD, Titball RW. Nucleotide sequence of the NS5 gene of Banzi virus: comparison with other flaviviruses. J Gen Virol. 1995;76(Pt 9):2317–21. doi: 10.1099/0022-1317-76-9-2317. [DOI] [PubMed] [Google Scholar]
- Gowen BB, Barnard DL, Smee DF, Wong MH, Pace AM, Jung KH, Winslow SG, Bailey KW, Blatt LM, Sidwell RW. Interferon alfacon-1 protects hamsters from lethal Pichinde virus infection. Antimicrob Agents Chemother. 2005;49(6):2378–86. doi: 10.1128/AAC.49.6.2378-2386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Satake Y, Kobayashi Y. Effect of cytokines on Japanese encephalitis virus production by human monocytes. Microbiol Immunol. 1990;34(5):459–66. doi: 10.1111/j.1348-0421.1990.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Ireland DD, Palian BM, Reiss CS. Interleukin (IL)-12 receptor beta1 or IL-12 receptor beta2 deficiency in mice indicates that IL-12 and IL-23 are not essential for host recovery from viral encephalitis. Viral Immunol. 2005;18(2):397–402. doi: 10.1089/vim.2005.18.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RO, Bhatt PN. Genetic resistance to lethal flavivirus encephalitis. I. Infection of congenic mice with Banzi virus. J Infect Dis. 1976;134(2):158–65. doi: 10.1093/infdis/134.2.158. [DOI] [PubMed] [Google Scholar]
- Leyssen P, Drosten C, Paning M, Charlier N, Paeshuyse J, De Clercq E, Neyts J. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob Agents Chemother. 2003;47(2):777–82. doi: 10.1128/AAC.47.2.777-782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antiviral Chemistry and Chemotherapy. 2004;15:67–75. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- Pantelic L, Sivakumaran H, Urosevic N. Differential induction of antiviral effects against West Nile virus in primary mouse macrophages derived from flavivirus-susceptible and congenic resistant mice by alpha/beta interferon and poly(I-C) J Virol. 2005;79(3):1753–64. doi: 10.1128/JVI.79.3.1753-1764.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AJ, Morahan PS, Brinton M, Stewart D, Gavin E. Comparative therapeutic efficacy of recombinant interferons-alpha, -beta, and -gamma against alphatogavirus, bunyavirus, flavivirus, and herpesvirus infections. J Interferon Res. 1990;10(3):293–8. doi: 10.1089/jir.1990.10.293. [DOI] [PubMed] [Google Scholar]
- Pinto AJ, Morahan PS, Brinton MA. Comparative study of various immunomodulators for macrophage and natural killer cell activation and antiviral efficacy against exotic RNA viruses. Int J Immunopharmacol. 1988;10(3):197–209. doi: 10.1016/0192-0561(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Guedes F, Takakura CF, Andrade HF, Jr., Vasconcelos PF, Duarte MI. Revisiting the liver in human yellow fever: virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology. 2006;345(1):22–30. doi: 10.1016/j.virol.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Rollier C, Verschoor EJ, Paranhos-Baccala G, Drexhage JA, Verstrepen BE, Berland JL, Himoudi N, Barnfield C, Liljestrom P, Lasarte JJ, Ruiz J, Inchauspe G, Heeney JL. Modulation of vaccine-induced immune responses to hepatitis C virus in rhesus macaques by altering priming before adenovirus boosting. J Infect Dis. 2005;192(5):920–9. doi: 10.1086/432517. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, Juckett DA, Aylsworth CF, Dimitrov NV, Ho SC, Judge JW, Kessel S, Quensen J, Wong KP, Zlatkin I, Zlatkin T. Protein from intestinal Eimeria protozoan stimulates IL-12 release from dendritic cells, exhibits antitumor properties in vivo and is correlated with low intestinal tumorigenicity. Int J Cancer. 2005;114(5):756–65. doi: 10.1002/ijc.20801. [DOI] [PubMed] [Google Scholar]
- Singh IP, Coppenhaver DH, Sarzotti M, Sriyuktasuth P, Poast J, Levy HB, Baron S. Postinfection therapy of arbovirus infections in mice. Antimicrob Agents Chemother. 1989;33(12):2126–31. doi: 10.1128/aac.33.12.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Alaghamandan HA, Gilbert J, Burger RA, Jin A, Sharma BS, Ramasamy K, Revankar GR, Cottam HB, Jolley WB. Immunoenhancing properties and antiviral activity of 7-deazaguanosine in mice. Antimicrob Agents Chemother. 1991;35(1):152–7. doi: 10.1128/aac.35.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Alaghamandan HA, Ramasamy K, Revankar GR. Broad-spectrum activity of 8-chloro-7-deazaguanosine against RNA virus infections in mice and rats. Antiviral Res. 1995;26(2):203–9. doi: 10.1016/0166-3542(94)00084-L. [DOI] [PMC free article] [PubMed] [Google Scholar]