Abstract
The polyomavirus enhancer binding protein 2αB (AML1/PEBP2αB/Cbfa2) plays a pivotal role in granulocyte colony-stimulating factor (G-CSF)-mediated differentiation of a myeloid progenitor cell line, 32Dc13. In this article, we report the identification of a PEBP2αB interacting protein, Ear-2, an orphan member of the nuclear hormone receptor superfamily that directly binds to and can inhibit the function of PEBP2αB. Ear-2 is expressed in proliferating 32Dc13 cells in presence of interleukin 3 but is down-regulated during differentiation induced by G-CSF. Interestingly, AML1/ETO(MTG8), a leukemogenic chimeric protein can block the differentiation of 32Dc13 cells, which is accompanied by the sustained expression of ear-2. Overexpression of Ear-2 can prevent G-CSF-induced differentiation, strongly suggesting that ear-2 is a key negative regulator of granulocytic differentiation. Our results indicate that a dynamic balance existing between PEBP2αB and Ear-2 appears to determine the choice between growth or differentiation for myeloid cells.
The granulocyte colony stimulating factor (G-CSF) is a major cytokine capable of generating granulocytes in the body to help prevent bacterial infection. Clinically, administration of G-CSF has been shown to be very effective in increasing the number of granulocytes in cases of deficiency. In addition to promoting granulocytic differentiation, G-CSF also stimulates the growth of various leukemic and other cells (for review, see refs. 1 and 2). It is, therefore, of great importance to clarify the mechanisms by which signals from the G-CSF receptor stimulate cell growth or terminal differentiation.
The murine myeloid progenitor cell line 32Dcl3 requires interleukin 3 (IL-3) for growth and undergoes granulocytic differentiation when IL-3 is replaced by G-CSF (3). It has been suggested that the function of the acute myeloid leukemia gene 1 (AML1) is required for G-CSF-induced differentiation (4, 5). AML1 [also called polyomavirus enhancer binding protein 2 αB gene (PEBP2αB) or core binding factor A2 gene (CBFA2)] is one of the three mammalian Runt-domain-encoding genes and its product is the α subunit of a heterodimeric transcription factor PEBP2/CBF (for review, see refs. 6 and 7). PEBP2/CBF containing AML1 as an α subunit is required for the onset of definitive hematopoiesis (8–12) and for the regulation of many hematopoietic-cell-specific genes such as IL-3, granulocyte-macrophage colony stimulating factor, T cell receptors α, β, γ, and δ (for review, see refs. 6 and 7), granzyme B (13), and bcl-2 (14). It is the most frequent target of chromosome translocations associated with human acute leukemia of myeloid (for review, see ref. 15) and lymphoid origins (16–18). The most commonly occurring chromosome translocation associated with acute myeloid leukemia is t(8;21), which generates a chimeric protein, AML1/ETO(MTG8). This fusion protein contains the amino-terminal region of AML1 up to the Runt domain, which harbors the DNA binding and dimerization activities, fused to almost the entire ETO(MTG8) protein (19, 20). AML1/ETO(MTG8) acts dominantly over AML1 and contributes to leukemogenesis by inhibiting the terminal differentiation of cells in myeloid lineage and maintaining cell growth (21). Some of the many reasons for studying G-CSF-induced differentiation of 32Dcl3 cells are to deduce the mechanism by which G-CSF induces differentiation, to clarify the role of AML1 in the process of differentiation, and to understand how AML1/ETO(MTG8) perturbs the normal process of differentiation.
In this paper, we report that the expression of ear-2 (erbA related gene), a member of the nuclear hormone receptor superfamily (22–24), is down-regulated in 32Dcl3 cells upon stimulation by G-CSF. We found that exogenous expression of ear-2 or AML1/ETO(MTG8) prevented G-CSF-induced differentiation in 32Dcl3 cells and that the product of Ear-2 directly interacts with AML1 and inhibits its activity. In flies, seven-up, Drosophila homolog of ear-2, has been shown to be negatively regulated by lozenge, a Runt-domain-encoding homolog of AML1, in photoreceptor R7 and in non-neuronal cells. Additionally, a gain-of-function mutation of lozenge, which misexpresses the gene product in R3 and R4, would repress the expression of seven-up in these cells and convert them to photoreceptor R7 (25). Herein we study the roles and mutual relationship of the mammalian homologs of these genes in hematopoietic cell differentiation.
MATERIALS AND METHODS
Plasmid Construction.
For expression of PEBP2αB1 (full-length mouse AML1 encoding 451 amino acids) in yeast, the 1.5-kb EcoRI fragment of pKS-PEBP2αB1 (26) was subcloned into a yeast expression vector, pGBT9 (CLONTECH), after modification of the ATG region to obtain a correct reading frame with the yeast expression vector. A series of deletion mutants of PEBP2αB1 was constructed with the site-directed mutagenesis kit (CLONTECH). Because the ear-2 cDNA obtained with the yeast two-hybrid system lacked part of the 5′ coding region, the missing portion (encoding 1–178 amino acids) was generated by PCR using a T cell cDNA library (CLONTECH) as template and subcloned into the XhoI sites of pKS (pKS-ear-2). The bacterial expression plasmid pGEX-5X-3-Ear-2 was constructed by inserting the XhoI fragment of pKS-ear-2 into the cognate sites of pGEX-5X-3 (Pharmacia). For in vitro translation, pKS-PEBP2αB1 (26) and C-terminal deletion mutants of PEBP2αB1, pKS-PEBP2αB1(1–331), pKS-PEBP2αB1(1–242), and pKS-PEBP2αB1(1–173) were generated as described (26). N-terminal deletion mutants of PEBP2αB1, pCITE-PEBP2αB1(174–451), pCITE-PEBP2αB1(179–343), pCITE-PEBP2αB1(349–451), and pCITE-PEBP2αB1(263–343) were constructed by using the PCR method and then subcloned into the EcoRI–XhoI sites of pCITE4 (Novagen). Mammalian expression vectors pEF-αB1 and pEF-β2 were as described (26). For mammalian expression of ear-2, the EcoRI–BssHII fragment of pKS-ear-2 was subcloned into the EcoRI–SmaI sites of the pCMX vector. MSV-C/EBPα and the reporter M-CSF-R-luc were a gift from Dong Er Zhang (27). For establishment of 32Dcl3 stable transfectants, a retrovirus construct, pBabeneo-ear-2, was generated from pcDNA3.1/HisA-ear-2. pcDNA3.1/HisA contains an Anti-Xpress-antibody epitope (Invitrogen). pcDNA3.1/HisA-ear-2 was generated by inserting the XhoI fragment of pKS-ear-2 into the cognate site of pcDNA3.1/HisA. The HindIII–XbaI fragment containing the Anti-Xpress-antibody epitope was inserted into the SnaBI site of the pBabeneo retrovirus vector after blunt-end treatment. The retroviral pBabeneo-AML1/ETO construct was generated by inserting the blunt-ended NotI–SalI fragment of pSK-AML1/ETO into the SnaBI site of pBabeneo.
Yeast Two-Hybrid Screens.
The GAL4 DNA-binding domain–PEBP2αB1 fusion construct pGBT9-PEBP2αB1 was transformed into the yeast strain HF7c, which contains integrated GAL1UAS-lacZ and GAL1UAS-HIS3 reporter genes (CLONTECH). A mouse T cell lymphoma cDNA library constructed from C57 Black Kaplan T cell lymphoma (V13 cell line) was obtained from CLONTECH that uses the XhoI cloning site of a yeast expression vector pACT containing the GAL4 activation domain.
Binding of in Vitro-Synthesized 35S-Labeled Proteins to the Immobilized Glutathione S-Transferase (GST) Proteins.
[35S]Methionine-labeled in vitro-translated proteins were prepared by using the TNT system (Promega) from full-length PEBP2αB1, PEBP2αB1(1–331), PEBP2αB1(1–242), PEBP2αB1(1–173), PEBP2αB1(174–451), PEBP2αB1(179–343), PEBP2αB1(349–451), and PEBP2αB1(263–343) as templates. Programmed 35S-labeled reticulocyte lysates (2 μl of a total 50-μl reaction mixture) were analyzed by SDS/PAGE. Equal amounts of labeled in vitro-translated proteins were diluted to 200 μl of the reaction buffer containing 20 mM Hepes (pH 7.5), 50 mM KCl, 10% glycerol, 2 mM DTT, 0.2% BSA, and 0.2% Nonidet P-40. This sample was mixed with 20 μl of either GST- or GST-Ear-2-bound beads (1:1) and incubated for 5 h on a rotary shaker at 4°C. The resin was then washed five times at room temperature with washing buffer (reaction buffer containing 0.3% instead 0.2% Nonidet P-40). The bound proteins were eluted with loading buffer (50 mM Tris⋅HCl, pH 6.8/100 mM DTT/2% SDS/0.1% bromophenol blue/10% glycerol) and analyzed by SDS/PAGE on 15% gels and autoradiography.
Preparation of Whole Cell Extracts and Western Blot Analysis.
Whole cell extracts of 32Dcl3 cells were prepared by a freezing–thawing method as described (28). The extracts were analyzed by SDS/PAGE on 12.5% gels and Western blotting, as described (29) except that the ECL Western blot analysis system (Amersham) was used to visualize proteins.
Transient Transfection and Luciferase Assays.
Effector plasmids, M-CSF-R-luc reporter, and internal control plasmid pRSV-β-gal were cotransfected into 1 × 107 Jurkat T cells by using electroporation with the conditions of 250 V and 960 μF (Gene Pulser, Bio-Rad). The total amount of transfected plasmid DNAs was kept constant at 20 μg by adding appropriate amounts of the backbone plasmid. After 24 h of transfection, cells were harvested and luciferase activites were measured and normalized by β-galactosidase activities. All transfection experiments were performed at least three times.
Cells.
A murine IL-3-dependent myeloid progenitor cell line derived from normal bone-marrow long-term culture, 32Dcl3 (3), was maintained in RPMI 1640 medium (GIBCO/BRL) supplemented with 10% fetal bovine serum and 5% WEHI-3B (30) supernatant as a source of IL-3. A Jurkat human T cell leukemia cell line (31) and a murine fibroblast cell line for packaging recombinant murine retrovirus ϕ2 (32) were cultured in RPMI 1640 medium and DMEM, respectively, supplemented with 10% fetal bovine serum.
Establishment of 32Dcl3 Stable Transfectants.
32Dcl3 clones stably expressing ear-2 and AML1/ETO(MTG8) were isolated by using a retrovirus vector, pBabeneo, as described (5).
Northern Blot Analysis.
Total RNA was isolated by using TRIzol Reagent (GIBCO/BRL). Poly(A)+ RNA was prepared by using Oligo[d(T)]-latex (Takara). Poly(A)+ RNA (5 μg or 2.5 μg) was separated on 1% agarose/formaldehyde gel and transferred to nylon membrane Hybond N+ (Amersham) in 20× SSPE (0.18 M NaCl/10 mM sodium phosphate, pH 7.4/1 mM EDTA) and fixed by a UV crosslinker (Spectrolinker). The probes were labeled with [α-32P]dCTP by using the Rediprime system (Amersham). The blot was hybridized and washed as described (5). Membranes were exposed to x-ray film for autoradiography.
RESULTS
Interaction of AML1/PEBP2αB1 with Ear-2.
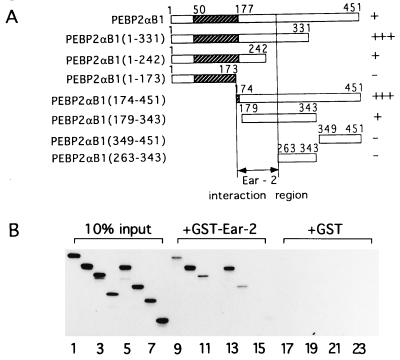
We had observed that multiple positive and negative factors interact with the C-terminal region of PEBP2αB1 [the 451-amino acid full-length murine AML1 will be referred to as PEBP2αB1 (26, 33) throughout this manuscript] and that these interactions appeared to be essential for both transcriptional activation and induction of 32Dcl3 cell differentiation (5). To identify such PEBP2αB1-interacting proteins, we used the yeast two-hybrid system and the entire murine PEBP2αB1 linked to the DNA binding domain of GAL4 as a bait. From 6 × 106 original transformants, 250 HIS+/Lac+ double-positive clones were isolated. One hundred clones were further selected by β-galactosidase assays in another yeast strain, SFY526, containing a GAL1UAS-LacZ reporter gene. Five percent of the positive clones represented the β subunit gene Pebpb2 (26). Of the remainder, 4 clones represented a gene previously identified and termed ear-2 (24). In this article, we studied biological significance of the interaction between PEBP2αB1 and Ear-2. Characterization of the remaining positive clones is under way. The minimum region present in the ear-2 cDNAs encoded the C-terminal region of Ear-2 known to be a putative ligand binding domain (amino acids 179–390). We attempted to localize the region of interaction by using a series of deletion constructs of PEBP2αB1 in yeast. Curiously, removal of 120 amino acids from the C terminus of PEBP2αB1 unveiled transactivational activity but removal of a further 129 amino acids abolished this activity. This essential 129-amino acid region includes a recently identified intrinsic transcriptional activation domain (T. Kanno and Y.I., unpublished results). However, when regions downstream of the Runt domain were linked directly to the DNA binding domain of GAL4, activity was not detected. With the latter constructs, we found that Ear-2 can interact with the region between amino acids 242- 262 (data not shown).
Direct interaction of PEBP2αB1 and Ear-2 in vitro was examined by GST pull-down assays using a series of deletion constructs of PEBP2αB1 (Fig. 1A). The results shown in Fig. 1B indicated involvement of the region of PEBP2αB1 between amino acids 173 and 262, which includes the Ear-2 binding domain identified above. In this case, the region between amino acids 173 and 242, which was negative in yeast two-hybrid assays, was shown to interact weakly. This discrepancy in the two assay systems is most likely due to difference in the structure of the constructs used: In the yeast two-hybrid assay, PEBP2αB1 fused to GAL4 DNA binding domain was used as a bait, whereas in the GST pull down assay, in vitro-translated PEBP2αB1 and its deletion derivatives were examined for interaction with GST–Ear-2. This proposed Ear-2 interacting region is missing in AML/ETO(MTG8).
Figure 1.
Physical interaction between PEBPαB1 and Ear2. (A) Full-length PEBPαB1 and its deletion derivatives are schematically shown. Stippled boxes represent the Runt domain. Degrees of interaction shown in B are summarized as −, +, and +++ on the right. (B) PEBPαB1 and its deletion derivatives were translated by programmed reticulocyte lysate in the presence of [35S]methionine. Ten percent of the material used in the GST pull down experiment is shown (lanes 1–8). In vitro-translated proteins were mixed with GST–Ear-2-bound beads (lanes 9–16) or the GST-bound beads (lanes 17–24), and a GST pull down assay was performed. Lanes: 1, 9, and 17, PEBP2αB1; 2, 10, and 18, PEBP2αB1(1–331); 3, 11, and 19, PEBP2αB1(1–242); 4, 12, and 20, PEBP2αB1(1–173); 5, 13, and 21, PEBP2αB1(174–451); 6, 14, and 22, PEBP2αB1(179–343); 7, 15, and 23, PEBP2αB1(349–451); 8, 16, and 24, PEBP2αB1(263–343). The proteins bound to the matrix were then analyzed by SDS/PAGE on 15% gels and autoradiography.
Inhibition of the PEBP2αB1 Activity by Ear-2 in Transcriptional Assays.
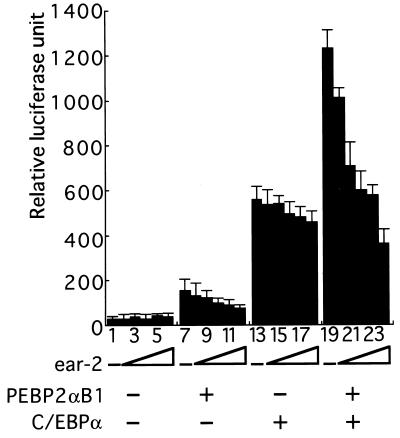
We examined whether Ear-2 could affect PEBP2αB1 activity by direct interaction. The macrophage-colony-stimulating factor receptor promoter is regulated by CCAAT enhancer binding protein (C/EBP), PU.1, and PEBP2/CBF (27, 34,). As shown in Fig. 2, C/EBPα stimulates this promoter activity by about 20-fold under the conditions used (Fig. 2, compare lanes 1 and 13). When PEBP2αB1 and the β subunit were coexpressed, the activity went up by 2-fold (Fig. 2, compare lanes 13 and 19). When increasing amounts of Ear-2 were added, progressive inhibition of the activity was observed (Fig. 2, lanes 19–24), although Ear-2 did not inhibit the basal promoter activity (Fig. 2, lanes 1–6). Preliminary results suggesting that Ear-2 inhibits transcription by binding to PEBP2αB1 have been obtained by the mutation analysis of the Ear-2 binding region of PEBP2αB1: PEBP2αB1 containing Ser → Ala and Tyr → Ala substitutions at amino acids 253 and 254 (S253A/Y254A) or the one containing S253A/Y254A/P251A/D255A had reduced ability to interact with Ear-2 in the yeast two-hybrid assay and enhanced activity to stimulate transcription in vivo. These mutants were less sensitive to the inhibitory effect of Ear-2 (data not shown). Ear-2 is a DNA binding protein but the reporter M-CSF-R-luc that we used contains no known Ear-2 binding sequence.
Figure 2.
Ear-2 represses transactivation mediated by PEBP2αB1. A Jurkat T cell line was transfected with 1 μg of MCSF-R-luc, 2 μg of pEF-PEBP2αB1, 2 μg of MSV-C/EBP, 1 μg of pEF-PEBP2β2, 0.5 μg of RSV-β-gal, and increasing amounts of pCMX-ear-2. Lanes: 1, 7, 13, and 19, 0 μg; 2, 8, 14, and 20, 1 μg; 3, 9, 15, and 21, 2 μg; 4, 10, 16, and 22, 4 μg; 5, 11, 17, and 23, 8 μg; 6, 12, 18, and 24, 12 μg. Luciferase activities were normalized to the β-galactosidase activities of each extract. Each value represents the mean of three independent experiments. Standard deviations are indicated by error bars.
Block of Differentiation of 32Dcl3 Cells by ear-2.
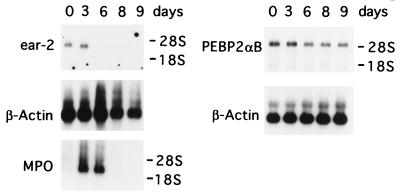
Because PEBP2αB is believed to be required for terminal differentiation of 32Dcl3 cells (4, 5) and Ear-2 inhibits PEBP2αB function, we decided to examine the expression of ear-2 in 32Dcl3 cells during differentiation (Fig. 3). Under the conditions used, 32Dcl3 cells continued to grow for about 5 days after replacement of IL-3 with G-CSF, then underwent gross morphological changes on or around day 6, and gradually lost viability afterward (5). Northern blot analyses revealed that the expression of ear-2 did not change appreciably in the first few days from the level observed in the presence of IL-3 but gradually decreased and after day 6, its expression was undetectable. On the contrary, PEBP2αB expression was virtually unaffected throughout this period. Considering the dramatic changes both in morphology and the pattern of gene expression in the cells during this period, this constant steady-state level of PEBP2αB RNA was surprising. By Western blot analysis, we confirmed that PEBP2αB protein was synthesized and that its level did not change appreciably during this period (data not shown). As reported (35), myeloperoxidase expression was induced at high levels after the G-CSF treatment (Fig. 3).
Figure 3.
Northern blot analysis of endogenous PEBP2αB1 and ear-2 mRNA in 32Dcl3 cells during granulocytic differentiation. Poly(A)+ RNAs were prepared from 32Dcl3 stably transfected with the pBabeneo vector alone at days 0, 3, 6, 8, and 9 after the addition of G-CSF. Five micrograms of poly(A)+ RNA was analyzed by Northern blotting using 32P-labeled ear-2 cDNA as a probe. To detect an early differentiation maker, MPO, its cDNA was used as a probe for the same blot after it was stripped. A different membrane containing 2.5 μg of poly(A)+ RNA was hybridized with 32P-labeled PEBP2αB1 cDNA as a probe. As an internal control, β-actin mRNA expression was analyzed for each membrane after striping. Positions of 18S and 28S rRNAs are indicated.
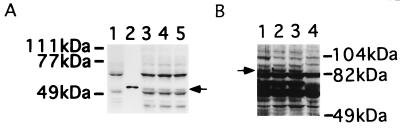
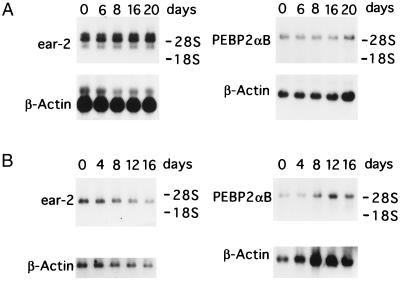
We observed an apparent correlation between the decrease of ear-2 expression and the differentiation of 32Dcl3 cells. To further investigate the possibility that ear-2 may be a negative regulator of granulocytic differentiation, clones of 32Dcl3 cells stably expressing ear-2 were isolated. Fig. 4A confirms the synthesis of exogenous Ear-2 by three independent clones of 32Dcl3 cells, which is not detected in the clone receiving only vector. Two clones of Ear-2 expressing cells were chosen and their growth curves were compared with control cells that received vector alone (Fig. 5). Both clones of 32Dcl3 that expressed exogenous ear-2 did not respond to G-CSF by differentiating and continued to grow in the presence of G-CSF. Furthermore, the cells could not survive when G-CSF was removed from the medium (data not shown), suggesting that in the presence of Ear-2, the cells use G-CSF as a growth factor. Exogenous expression of ear-2 was significantly higher than the level of the endogenous gene expression (the lowest of the three bands in Fig. 6A), which similar to PEBP2aB expression was maintained at constant levels over the 20-day period of cell growth (Fig. 6A). These results suggested that expression of ear-2 is not autoregulated and that expression of PEBP2aB is not controlled by ear-2. Ear-2 did not appreciably change the IL-3 or serum requirement of 32Dcl3 cells (data not shown). These results indicate that ear-2 negatively regulates granulocytic differentiation in response to G-CSF.
Figure 4.
Western blot analysis of Ear-2 or AML1/ETO(MTG8) synthesized in 32Dcl3 cells infected with retrovirus expressing pBabeneo-ear-2 or AML1/ETO(MTG8), respectively. (A) Twenty micrograms of whole cell extracts from pBabeneo vector transfected clone (lane 1) and pBabeneo-ear-2 transfected clones (lanes 3–5) were subjected to Western blot analysis with anti-Xpress antibody (CLONTECH). An arrow indicates the Ear-2 band. In vitro-translated Ear-2 was used as a marker (lane 2). (B) Twenty micrograms of whole cell extracts of 32Dcl3 cells transfected with pBabeneo-AML1/ETO(MTG8) was analyzed by using anti-PEBP2αB1 (lanes 1–3). An arrow indicates the AML1/ETO(MTG8) band. A 32Dcl3 clone transfected with pBabeneo vector alone was used as a control (lanes 4).
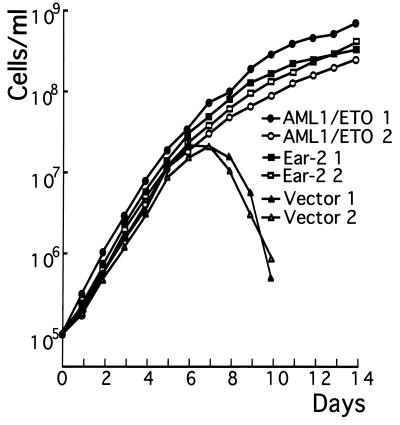
Figure 5.
Growth curve of 32Dcl3 cells in response to G-CSF. Approximately 1 × 106 cells of ear-2 or AML1/ETO(MTG8)-expressing clones or those received only the vector growing in the presence of IL-3 were washed twice with PBS and cultured in the medium containing recombinant human G-CSF (500 units/ml). At each time point, viable cells excluding trypan blue were counted by using a hemocytometer. Two independent experiments were performed. ▪ and □, Ear-2 1 and Ear-2 2, respectively; • and ○, AML1/ETO(MTG8) 1 and AML1/ETO(MTG8) 2, respectively; ▴ and ▵, Vector 1 and Vector 2, respectively.
Figure 6.
Northern blot analysis of PEBP2αB1 and ear-2 mRNA. Poly(A)+ RNAs from 32Dcl3 cells stably transfected with pBabeneo-ear-2 (A) or pBabeneo-AML1/ETO(MTG8) (B) were analyzed by Northern blotting using 5 μg or 2.5 μg of poly(A)+ RNA for the expression of ear-2 or PEBP2αB, respectively. Numbers indicate days in the medium containing G-CSF. Of the three bands representing the ear-2 mRNA in A, top two correspond to the exogenous and the bottom one to the endogenous mRNA. As an internal control, β-actin mRNA expression was analyzed for each membrane after stripping. The positions of 18S and 28S rRNA are indicated. AML1/ETO(MTG8) RNA was not detected by the labeled PEBP2αB1 probe in B Right. Expression of AML1/ETO(MTG8) was confirmed, however, by using the AML1/ETO(MTG8) cDNA as a probe (data not shown).
Block of Differentiation by AML1/ETO(MTG8).
The chimeric gene AML1/ETO(MTG8) functions dominantly over AML1 and blocks differentiation of Kasumi-1 cells harboring t(8;21) obtained from an acute myeloid leukemia patient (21). It does not have the region of AML1 responsible for transactivation and behaves as a dominant interfering molecule in transcription assays (36). When this chimeric protein was exogenously expressed in 32Dcl3 cells (Fig. 4B), granulocytic differentiation was also blocked in both of the clones tested (Fig. 5).
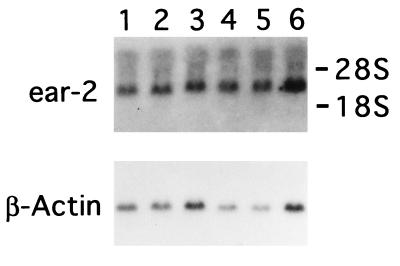
A possible mechanism by which AML1/ETO(MTG8) blocks differentiation of 32Dcl3 cells could be by enhancing ear-2 expression or inhibiting its down-regulation. Levels of endogenous ear-2 expression in 32Dcl3 cells exogenously expressing AML1/ETO(MTG8) were examined in four independent clones in the presence of IL-3. As shown in Fig. 7, the levels of expression were comparable to those of control cells in all cases. However, expression of endogenous ear-2 was not down-regulated by G-CSF in the cells exogenously expressing AML1/ETO(MTG8) during the period extending to 16 days after G-CSF treatment (Fig. 6B). Endogenous PEBP2αB expression was also unchanged (Fig. 6B). AML1/ETO(MTG8) transcripts that did not cross-hybridize with the PEBP2αB cDNA probe were detected in these clones with a labeled AML1/ETO(MTG8) probe (data not shown).
Figure 7.
Northern blot analysis of endogenous ear-2 mRNA in the cell clones expressing AML1/ETO(MTG8). Poly(A)+ RNAs from parental 32Dcl3 cells (lane 1), 32Dcl3 cell clone stably transfected with pBabeneo vector alone (lane 2), or 32Dcl3 cell clones stably transfected with pBabeneo-AML1/ETO(MTG8) (lanes 3–6), which were cultured in the medium containing IL-3, were analyzed by using 5 μg of poly(A)+ RNA and ear-2 cDNA as a probe. β-actin mRNA is shown as an internal control. The positions of 18S and 28S rRNA are indicated.
DISCUSSION
In this paper, we described three major observations: (i) ear-2, a mammalian homolog of Drosophila seven-up, prevents differentiation of 32Dcl3 cells in the presence of G-CSF when it is expressed above certain levels, (ii) Ear-2 inhibits the transcriptional activation function of murine AML1/PEBP2αB1 apparently by directly binding to it, and (iii) AML1/ETO(MTG8) inhibits G-CSF-induced differentiation of 32Dcl3 cells.
It has been shown that ErbA abrogates the dependency of chicken embryo fibroblasts on serum growth factors (37). For another member of the nuclear hormone receptor family, Ear-2, we observed a similarly striking yet distinct effect on cells. 32Dcl3 cells undergo terminal differentiation or continue to grow when they are treated with G-CSF, depending on the levels of ear-2 expression. In other words, intracellular signals elicited from the G-CSF receptor appear to be converted to either a growth signal or a differentiation signal, depending upon the concentration of Ear-2 in the cells. However, in either case, Ear-2 could not eliminate the necessity of G-CSF for cell survival.
We showed that Ear-2 directly interacts with PEBP2αB1 in vivo and in vitro and inhibits its ability to transactivate in transfected cells. These results and earlier observations that AML1/PEBP2αB is required for induction of granulocytic differentiation (4, 5) suggest the possibility that Ear-2 and AML1/PEBP2αB function together in controlling granulocytic differentiation: the decision to grow or to differentiate may be determined by a combinatorial action of Ear-2 and PEBP2αB1. We attempted to obtain more direct evidence to support the hypotheses that the main in vivo target of Ear-2 is actually PEBP2αB1. We synthesized oligopeptides representing the region of PEBP2αB1 responsible for Ear-2 interaction and examined whether these oligopeptides could inhibit the Ear-2 effect. Although we observed some effects on differentiation after the addition of the peptide, we found subsequently that more than one protein could interact with PEBP2αB1 between amino acids 173 and 262. We are therefore unable to make a definitive conclusion until we precisely analyze the interaction domains for all of these proteins. If indeed the main target of Ear-2 is AML1/PEBP2αB1 in vivo, we propose the following scenario. When the cells are growing in the presence of IL-3, the PEBP2αB protein may be largely inactivated by Ear-2. Upon G-CSF stimulation, Ear-2 is down-regulated but the synthesis of PEBP2αB is maintained and PEBP2αB becomes active. In this case, Ear-2 would be an obligatory partner of PEBP2αB1 in determining the level of the PEBP2αB1 activity.
ear-2 did not affect the expression of PEBP2αB. Likewise PEBP2αB does not appear to be primarily involved in regulation of ear-2 expression, because AML1/ETO(MTG8), a dominant negative regulator of AML did not alter appreciably the expression of ear-2. Expression of ear-2 persists in 32Dcl3 cells expressing AML1/ETO(MTG8) even after stimulation by G-CSF. This could be explained by the involvement of PEBP2αB in regulation of a gene(s) required for down-regulation of ear-2. Expression of this gene(s) should be responsive to stimulation by G-CSF.
The G-CSF receptor (G-CSF-R), upon stimulation by G-CSF, induces multiple signals from the cytoplasmic domains (1). The region proximal to the plasma membrane of the cytoplasmic domain of G-CSF-R is considered to be sufficient to elicit mitogenic signals. On the other hand, the distal portion, containing multiple tyrosine residues is believed to be required for the induction of differentiation (1). The proximal domain of G-CSF-R is responsible for activating STAT3 and STAT1 transcription factors. Recently, however, it was shown that blocking the STAT3 activity by a dominant negative interfering molecule inhibits differentiation (38). It is therefore of interest to study whether STAT3 activity is involved in the inhibition of ear-2 expression. However, the effect of G-CSF stimulation on STAT3 activity takes places within minutes, whereas its effect on ear-2 expression requires several days. Clearly, multiple steps are likely to be involved through which signals from G-CSF-R eventually regulate ear-2 expression.
Finally, it is worth examining whether some of the patterns of gene regulation observed in Drosophila eye development are conserved in mammalian hematopoietic cell differentiation. First, it would be interesting to see whether Ear-2 and Lozenge can directly interact. seven-up is involved in control of cell fate during the generation of neuronal diversity. The pattern of its expression has been described (39). The genes seven-up and lozenge are expressed simultaneously in photoreceptors R1 and R6 (U. Banerjee, personal communication), but expression of seven-up is inhibited if lozenge is misexpressed in R3 and R4 (25). Therefore, regulation of seven-up expression by lozenge appears dependent on cell context. There are several more genes now known to be involved in regulation of or that are regulated by these two genes. In 32Dcl3 cells, we did not obtain evidence that expression of ear-2 is directly regulated by AML1/PEBPαB. It is quite likely that many additional factors are involved in the process of granulocytic differentiation. It is clear to us that this is just the beginning and that we must go on to identify multiple presumptive factors and characterize the relationships among them before full understanding of the mechanisms of granulocytic differentiation and leukemogenesis caused by malfunction of the processes can be obtained.
Acknowledgments
We thank Alan D. Friedman (Johns Hopkins University) for 32Dcl3 cell line; Dong Er Zhang (Beth Israel Hospital) for MSV-C/EBPα and MCSF-R-luc; Takumi Era (Osaka University) for AML1/ETO cDNA; Junji Yodoi (Kyoto University) for Jurkat cells; and Tomohiko Kanno for his advice on transcription assay. We are indebted to Drs. Utpal Banerjee (University of California, Los Angeles) and Yasushi Hiromi (National Institute of Genetics, Japan) for discussions on Drosophila eye development. This work was partly supported by the Human Frontier Science Program (RG-357/94) and the New Energy and Industrial Technology Development Organization.
ABBREVIATIONS
- PEBP2αB
polyomavirus enhancer binding protein 2 αB
- G-CSF
granulocyte colony-stimulating factor
- IL-3
interleukin 3
- GST
glutathione S-transferase
- G-CSF-R
G-CSF receptor
References
- 1.Avalos B R. Blood. 1996;88:761–777. [PubMed] [Google Scholar]
- 2.Murakami H, Nagata S. In: The Cytokine Handbook. 3rd Ed. Thomson A W, editor. London: Academic; 1997. , in press. [Google Scholar]
- 3.Valtieri M, Tweardy D J, Caracciolo D, Johnson K, Mavilio F, Altmann S, Santoli D, Rovera G. J Immunol. 1987;138:3829–3835. [PubMed] [Google Scholar]
- 4.Tanaka T, Tanaka K, Ogawa S, Kurokawa M, Mitani K, Nishida J, Shibata Y, Yazaki Y, Hirai H. EMBO J. 1995;14:341–350. doi: 10.1002/j.1460-2075.1995.tb07008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y W, Bae S C, Huang G, Fu Y X, Lu J, Ahn M Y, Kanno Y, Kanno T, Ito Y. Mol Cell Biol. 1997;17:4133–4145. doi: 10.1128/mcb.17.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito Y. J Cancer Res Clin Oncol. 1996;122:266–274. doi: 10.1007/BF01261402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Bae S C. In: Cell Cycle Regulators and Chromosomal Translocation. Yaniv M, Ghysdael J, editors. Vol. 2. Basel: Birkhauser; 1997. pp. 107–132. [Google Scholar]
- 8.Niki M, Okada H, Takano H, Kuno J, Tani K, Hibino H, Asano S, Ito Y, Satake M, Noda T. Proc Natl Acad Sci USA. 1997;94:5697–5702. doi: 10.1073/pnas.94.11.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda T, Deursen J V, Hiebert S W, Grosveld G, Downing J R. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki K, Yagi H, Bronson R T, Tominaga K, Matsunashi T, Deguchi K, Tani Y, Kishimoto T, Komori T. Proc Natl Acad Sci USA. 1996;93:12359–12363. doi: 10.1073/pnas.93.22.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe A H, Speck N A. Proc Natl Acad Sci USA. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Stacy T, Miller J D, Lewis A F, Gu T L, Huang X, Bushweller J H, Bories J C, Alt F W, Ryan G, Liu P P, Wynshaw-Boris A, Binder M, Marin-Padilla M, Sharpe A H, Speck N A. Cell. 1996;87:697–708. doi: 10.1016/s0092-8674(00)81389-6. [DOI] [PubMed] [Google Scholar]
- 13.Wargnier A, Legros-Maida S, Bosselut R, Bourge J F, Lafaurie C, Ghysdael J, Sasportes M, Paul P. Proc Natl Acad Sci USA. 1995;92:6930–6934. doi: 10.1073/pnas.92.15.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klampfer L, Zhang J, Zelenets A O, Uchida H, Nimer S D. Proc Natl Acad Sci USA. 1996;93:14059–14064. doi: 10.1073/pnas.93.24.14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nucifora G, Rowley J D. Blood. 1995;86:1–14. [PubMed] [Google Scholar]
- 16.Golub T R, Barker G F, Bohlander S K, Hiebert S W, Ward D C, Bray-Ward P, Morgan E, Raimondi S C, Rowley J D, Gilliland D G. Proc Natl Acad Sci USA. 1995;92:4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romana S P, Mauchauffé M, Le Coniat M, Chumakov I, Le Paslier D, Berger R, Bernard O A. Blood. 1995;85:3662–3670. [PubMed] [Google Scholar]
- 18.Shurtleff S A, Buijs A, Behm F G, Rubnitz J E, Raimondi S C, Hancock M L, Chan G C, Pui C H, Grosveld G, Downing J R. Leukemia. 1995;9:1985–1989. [PubMed] [Google Scholar]
- 19.Erickson P, Gao J, Chang K S, Look T, Whisenant E, Raimondi S, Lasher R, Trujillo J, Rowley J, Drabkin H. Blood. 1992;80:1825–1831. [PubMed] [Google Scholar]
- 20.Miyoshi H, Kozu T, Shimizu K, Enomoto K, Maseki N, Kaneko Y, Kamada N, Ohki M. EMBO J. 1993;12:2715–2721. doi: 10.1002/j.1460-2075.1993.tb05933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakakura C, Yamaguchi-Iwai Y, Satake M, Bae S C, Takahashi A, Ogawa E, Hagiwara A, Takahashi T, Murakami A, Makino K, Nakagawa T, Kamada N, Ito Y. Proc Natl Acad Sci USA. 1994;91:11723–11727. doi: 10.1073/pnas.91.24.11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnhart K M, Mellon P L. Gene. 1994;142:313–314. doi: 10.1016/0378-1119(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 23.Ladias J A, Hadzopoulou-Cladaras M, Kardassis D, Cardot P, Cheng J, Zannis V, Cladaras C. J Biol Chem. 1992;267:15849–15860. [PubMed] [Google Scholar]
- 24.Miyajima N, Kadowaki Y, Fukushige S, Shimizu S, Semba K, Yamanashi Y, Matsubara K, Toyoshima K, Yamamoto T. Nucleic Acids Res. 1988;16:11057–11074. doi: 10.1093/nar/16.23.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daga A, Karlovich C A, Dumstrei K, Banerjiee U. Genes Dev. 1996;10:1194–1205. doi: 10.1101/gad.10.10.1194. [DOI] [PubMed] [Google Scholar]
- 26.Bae S C, Ogawa E, Maruyama M, Oka H, Satake M, Shigesada K, Jenkins N A, Gilbert D J, Copeland N G, Ito Y. Mol Cell Biol. 1994;14:3242–3252. doi: 10.1128/mcb.14.5.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang D E, Fujioka K, Hetherington C J, Shapiro L H, Chen H M, Look A T, Tenen D G. Mol Cell Biol. 1994;14:8085–8095. doi: 10.1128/mcb.14.12.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagano M, Durst M, Joswig S, Draetta G, Jansen-Durr P. Oncogene. 1992;7:1681–1686. [PubMed] [Google Scholar]
- 29.Lu J, Maruyama M, Satake M, Bae S C, Ogawa E, Kagoshima H, Shigesada K, Ito Y. Mol Cell Biol. 1995;15:1651–1661. doi: 10.1128/mcb.15.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner N L, Moore M A, Metcalf D. J Natl Cancer Inst. 1969;43:963–982. [PubMed] [Google Scholar]
- 31.Hansen J A, Martin P J, Nowinski R C. Immunogenetics. 1980;10:247–260. doi: 10.1007/BF01567812. [DOI] [PubMed] [Google Scholar]
- 32.Mann R, Mulligan R C, Baltimore D. Cell. 1983;33:153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- 33.Bae S C, Yamaguchi-Iwai Y, Ogawa E, Maruyama M, Inuzuka M, Kagoshima H, Shigesada K, Satake M, Ito Y. Oncogene. 1993;8:809–814. [PubMed] [Google Scholar]
- 34.Zhang D E, Zhang P, Wang N D, Hetherington C J, Darlington G J, Tenen D J. Proc Natl Acad Sci USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukunaga R, Ishizaka-Ikeda E, Nagata S. Cell. 1993;74:1079–1087. doi: 10.1016/0092-8674(93)90729-a. [DOI] [PubMed] [Google Scholar]
- 36.Myers S, Lenny N, Hiebert S W. Mol Cell Biol. 1995;15:1974–1982. doi: 10.1128/mcb.15.4.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandrillon O, Jurdic P, Benchaibi M, Xiao J H, Ghysdael J, Samarut J. Cell. 1987;49:687–697. doi: 10.1016/0092-8674(87)90545-9. [DOI] [PubMed] [Google Scholar]
- 38.Shimozaki K, Nakajima K, Hirano T, Nagata S. J Biol Chem. 1997;272:25184–25189. doi: 10.1074/jbc.272.40.25184. [DOI] [PubMed] [Google Scholar]
- 39.Mlodziki M, Hiromi Y, Weber U, Goodman C S, Rubin G M. Cell. 1990;60:211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]